Uniportal video-assisted thoracoscopic lobectomy
Introduction
The development of video assisted thoracic surgery (VATS) has been the single greatest advance in thoracic surgery of this generation (1,2). VATS promises less morbidity and faster recovery compared to open thoracotomy (3,4). In recent years, VATS has evolved into new, innovative approaches, including needlescopic and 2-port VATS. However, the uniportal approach confers the least invasiveness with only one single incision (5,6). This article focuses specifically on the technical aspects of how to perform the quintessential uniportal lobectomy.
Preparation
Patient selection and pre-operative preparation for uniportal VATS is no different from conventional VATS (1,2). Although variations exist in different institutions, the same principles of patient selection and pre-operative management for conventional VATS can be employed unchanged.
The patient is put under general anesthesia and one-lung ventilation is instituted. This author routinely requests pre-emptive regional blockade using a paravertebral bolus injection of a long-acting local anesthetic to achieve virtual absence of postoperative pain (4,7). More recently, the non-intubated technique for anesthesia has been described for uniportal VATS (8,9).
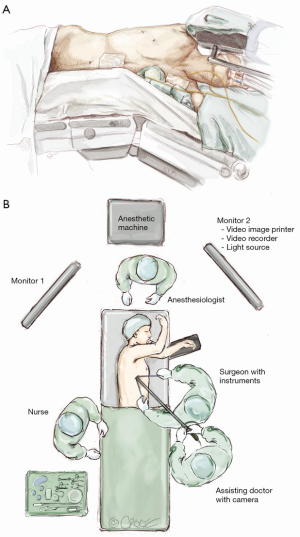
The patient is positioned as for conventional VATS (Figure 1). Since the uniport is sited relatively anteriorly, it is more ergonomic for the surgeon to stand in front of the patient who is turned laterally. The primary video monitor is placed opposite to the surgeon (i.e., behind the patient). The key is to ensure that the ‘axis’ of the operation (from the video-camera, through the wound, to the monitor) is kept in a straight line. When performing an upper lobectomy, the surgeon typically works in a more ‘feet-to-head’ direction and the monitor is best placed more towards the head end of the patient. When performing a lower lobectomy, the surgeon works slightly more towards the feet and the monitor may be better placed slightly more towards the patient’s feet.
This author strongly advises that instruments previously used for conventional VATS (or even open surgery) can still be used when learning the uniportal approach. Using familiar instruments and techniques shortens the learning curve. Specific equipment for uniportal VATS can be purchased subsequently when the surgeon’s experience may guide him or her in deciding which instruments are most suitable.
This author’s own preferences include:
- 30-degree 5 mm video-thoracoscope attached to a high-definition video camera system (this provides picture quality almost as good as a 10 mm scope but leaves more room within the small single port for fine instrumentation);
- Alexis-type wound protector (not necessary for all cases, but very useful when the assistant is inexperienced and repeated cleaning of the scope is required);
- Long, curved ring (sponge-holding) forceps for lung retraction;
- Long Metzenbaum scissors for dissection (more for spreading tissues than for direct cutting);
- Yankauer sucker for blunt dissection;
- Rumel and Roberts forceps for dissecting behind hilar structures;
- Long-tipped diathermy (electro-cautery) and/or ultrasonic dissection device for dissection;
- Endoscopic stapler (reloads with a curved tip on the anvil are expensive but can be very useful for negotiating vessels);
- Polymer vascular clips (as a cheaper alternative to stapling with smaller vessels).
It is prudent to establish criteria for conversion before proceeding. It should be decided beforehand how much time can be spent on any part of the operation (e.g., vein and artery dissection and adhesiolysis) or how much blood can be loss before conversion is mandatory. When those limits are reached, there should be no hesitation to convert.
Basic principles
Getting started
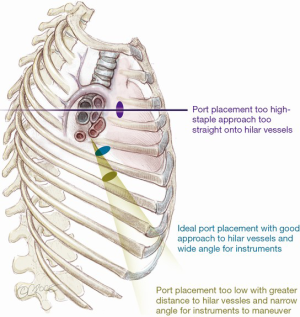
To take advantage of the naturally wider intercostal space, the uniport is sited relatively anteriorly on the chest (see below). As a result, it is more ergonomic to operate standing in front of the patient. Generally, the uniport is best sited between the mid- and anterior axillary lines in the 5th intercostal space (Figure 2). If the wound is sited too high in the 4th space for an upper lobectomy, dissection of the hilar vessels may be easier but the instruments enter so directly towards the hilum that there is insufficient angle for the stapler to pass without impinging on the structures behind. If the wound is too low, the angle to the hilar structures may be too acute and there may be considerable ‘fencing’ between the instruments and camera. With experience, the surgeon may experiment with other positions for the uniport but the beginner is advised to stick to this rule of thumb. The incision itself is typically 3–4 cm long. For larger tumors, this author has previously described a simple technique of cutting a rib anteriorly, allowing the intercostal space to open up without forceful rib spreading for large specimen retrieval (10). Using this technique, the author has delivered tumors over 8 cm in diameter through a 4–5cm incision. Ring forceps are used to grasp and retract the lung, taking care not to use excessive force which can tear the lung, leading to troublesome air leak.
Communicating with the assistant
During an operation, the surgeon cannot constantly stop and reposition the camera him or herself to achieve the correct view. It is therefore essential to have an effective means of verbally communicating the surgeon’s needs to the camera-wielding assistant.
Firstly, when the surgeon and assistant stand in front of the patient, the uniport virtually appears as a vertical keyhole slit (Figure 3A). A typical 3cm incision typically allows the placement of the video-thoracoscope, an instrument held by the surgeon’s right hand, and another instrument held by his or her left hand, one above another in a ‘traffic light’ configuration. The video-thoracoscope is kept in the uppermost ‘red light’ position, and the surgeon’s left and right hand instruments are inserted underneath in the ‘yellow’ and ‘green light’ positions. This is the ideal configuration to provide optimal visualization of the operating field. Keeping the video-thoracoscope pressed gently against the upper (posterior) end of the wound in the ‘red light’ position allows the assistant to rest it against a stable point, reducing wandering of the camera. With experience, the surgeon may occasionally request the assistant to place the video-thoracoscope in the ‘yellow’ or ‘green light’ positions in certain situations.

Second, the surgeon needs to verbally communicate how to maneuver the video-thoracoscope. The assistant should always keep the view level, with the ‘buttons’ on the camera head facing up towards the ceiling (Figure 3B). The surgeon can instruct the assistant to move the view in three directions: (I) physically moving the scope in or out to achieve a closer or more panoramic view; (II) moving the camera to look ‘up’, ‘down’, ’left’ or ‘right’ and (III) rotating the 30-degree lens to look around structures. To do the latter, the author uses the clock face image for communication. When using a conventional video-thoracoscope with a light cable attached to the scope from one side (Figure 3B), a ‘12 o’clock’ view means to hold the light cable from the top side of the video-thoracoscope, so that the 30 view is from the top looking downwards. A ‘3 o’clock view’ means to hold the light cable from the right side of the video-thoracoscope, so that the 30 view is from the right looking leftwards.
Operative technique (right upper lobectomy)
A right upper lobe (RUL) anatomical resection is ideal to showcase the tips and tricks for performing a uniportal lobectomy.
Pulmonary vein
The video-thoracoscope is used to inspect the thorax and any pleural adhesions are taken down. Contraindications to further surgery, such as. unexpected metastases, are excluded. If tissue diagnosis was not obtained prior to surgery, a biopsy of the primary lesion is taken and sent for frozen section analysis (11).
The first structure facing the surgeon in an anterior-to-posterior uniport approach is the superior pulmonary vein. The lung is retracted laterally and posteriorly to expose this vessel (Figure 4). The mediastinal pleura over the vein is opened and the fascia over the vein is dissected to the subadventitial layer using a combination of sharp and blunt instrumentation. Long Metzenbaum scissors for spreading and a Yankauer sucker for blunt dissection are ideal. It is essential to identify and preserve the vein from the right middle lobe.
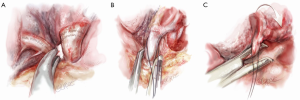
Curved forceps (e.g., long rumel clamp) are passed behind the vein and repeatedly opened and closed gently to open up that space behind. A stapler device is then passed. The thinner ‘anvil’ is engaged along the left side of the RUL pulmonary vein and the lung is then retracted forwards and in a cephalad direction. This movement opens up space behind the vein, between the vein and artery behind it. At the same time, the stapler is also gently rotated clockwise so that the reticulated head is not pointing straight into the hilum but instead in a more cephalad direction. In this direction, the stapler can be advanced left-to-right across the screen, and the anvil will avoid impacting on the structures behind. The stapler can be closed and fired. Using a roticulating stapler and a curved tip reload for the stapler makes stapling of the vein much easier, but each surgeon must decide individually whether the benefits outweigh the cost.
Pulmonary artery (truncus)
Once the vein is divided, the original retraction of the RUL laterally and posteriorly exposes the pulmonary artery behind where the vein once lay (Figure 4). The interlobar pulmonary artery runs almost horizontally across the monitor’s lower side from right to left. The RUL pulmonary artery truncus is seen running vertically (laterally) up from this to the RUL.
The truncus is dissected using sharp and blunt dissection in the same manner as with the pulmonary vein (Figure 5). Rumel curved forceps are again used to go behind the artery and a stapler is inserted with the thinner ‘anvil’ jaw engaging along the left side of the RUL pulmonary artery. The lung is then retracted forwards and in a cephalad direction to open up space behind the artery, between it and the bronchus behind. At the same time, the stapler is also very gently rotated clockwise so that the reticulated head is not pointing straight into the hilum but instead towards a more cephalad direction to avoid impinging onto the bronchus behind. The stapler should be fired steadily, avoiding rough, jerky movement which can tear or avulse the artery, which is gripped tightly by the stapler during firing.

If the truncus is not too large, polymer vascular ligating clips can be used instead of a stapler to cut costs. Two clips can be placed proximally. The distal end can be secured with another clip or sealed and divided using an energy device, such as an ultrasonic dissection-division device. Another method is to simply divide the vessel between ligatures as in traditional open surgery. Using a knot-pusher, this is entirely feasible with uniportal VATS.
Bronchus
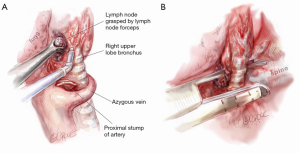
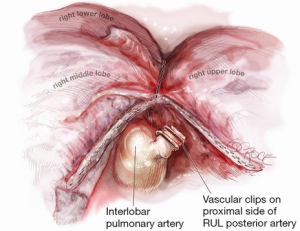
Once the artery is divided, the original retraction of the RUL laterally and posteriorly exposes the RUL bronchus behind where the truncus once stood (Figure 5). With this retraction, the RUL lobar bronchus rises vertically (laterally) up from the right main bronchus soon after it emerges from under the azygos vein.
The landmark to look for is the black interlobar lymph node invariably found to the left border of the RUL bronchus, demarcating it from the bronchus intermedius (Figure 5). This lymph node can be grasped and removed (Figure 6). Once the node is dissected away, either Roberts or Rumel forceps are passed along the left border of the RUL bronchus, looping around behind it. A stapler is inserted into the chest and the thinner ‘anvil’ jaw is inserted along the left side of the RUL bronchus. Occasionally, emergence of the stapler anvil may be blocked by impingement against the spine behind. If this occurs, the same trick of rotating both lung and forwards and in a cephalad direction is usually sufficient for the anvil to avoid the spine behind. The stapler should be fired only after testing for inflation of the middle and lower lobes on closing the staple.
It is possible to avoid use of the stapler by cutting the bronchus and then suturing the proximal stump. This is very occasionally needed for lobectomies requiring bronchoplasty or sleeve resection (12-14). However, for routine lobectomies, the cost saving may not be outweighed by the extra time and effort spent.
Pulmonary artery (posterior ascending branch)
Once the RUL bronchus is divided, the original retraction of the RUL laterally and posteriorly exposes any posterior ascending branch(es) of the pulmonary artery arising from the interlobar artery and supplying the RUL (Figure 7). With this retraction, the posterior ascending branch(es) rise vertically (laterally) up from the interlobar artery to enter the RUL.
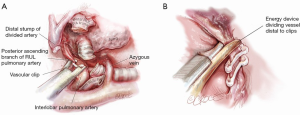
The posterior ascending branch(es) are dissected using sharp and blunt dissection in the same manner as with the pulmonary vein and pulmonary artery truncus (Figure 7). Roberts or Rumel curved forceps are again used to go around behind the branch(es) and very gentle opening and closing helps develop the space behind. The branch(es) can be divided with a stapler or between polymer vascular ligating clips. The latter are not only a means of cost saving but their applicators are often thinner than a stapler, reducing the risk of tearing vessels as may be caused by rough passage of a stapler.
Fissure
The lobectomy is completed by now detaching the RUL from the right middle and/or lower lobes. Leaving the fissure to be divided last with a stapler, the ‘fissureless’ (or ‘fissure last’) approach has been reported to result in fewer cases of air leak (4,15,16).
In some people, with very complete fissures, the fissures can sometimes be completed using an energy device (e.g., diathermy or an ultrasonic device). When doing so, the line of cutting should be kept slightly away from the remaining middle and lower lobes to reduce postoperative air leak from them. If the thickness of tissue in the fissures is too great, rather than risk significant air leak from dissection, it may be better to staple-divide them (Figure 8). Before firing the stapler, it is important to check that the stapling does not accidentally include major structures, such as the middle lobe pulmonary vein or the interlobar pulmonary artery. If polymer vascular clips had been used earlier in the operation, it is also necessary to ensure that none of these are caught in the stapler jaws before firing, as they can totally disrupt or prevent firing. Very often, the posterior ascending branch of the pulmonary artery to the RUL is very small. In some cases, some surgeons choose not to staple it together with completing the fissure.
Completion
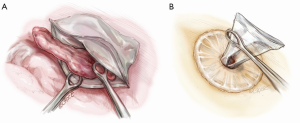
The resected lobe is placed inside a specimen bag before being delivered out of the uniport if malignancy has been confirmed or is suspected. This reduces the risk of tumor seeding at the wound and of squeezing tumor content into the pleural space as the tumor is pulled out of the small uniport. Dedicated endoscopic specimen retrieval bags are useful but expensive. Cheap, improvised alternatives include surgical gloves, intravenous fluid bags cut open on one side and others. This author uses a hospital specimen bag with a zip-lock opening which is sterilized in-house. The zip-lock ribbing allows the mouth of the bag to open up nicely inside the chest (Figure 9), allowing easy placement of the specimen into the bag. As noted above, for large tumors, an anterior rib-cutting technique can be used to allow delivery of even large specimens via a relatively small uniport (10).
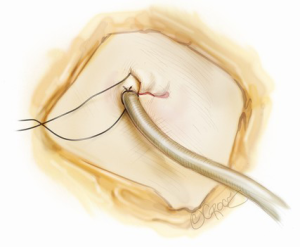
For all cases of malignancy, systematic lymph node dissection is mandatory. For any right-sided lobectomy, this author routinely dissects stations 2R, 3a, 4R, 7, 8R, 9R and 10R. The most common stations (4R and 7) are readily approachable by the single port approach. The pleural space is thoroughly lavaged with warm, sterile water. Air leak testing is conducted under water. This author uses flowable or topical hemostats liberally for any parenchymal air leaks detected as prolonged postoperative chest drainage may negate much of the benefit expected with the uniportal approach (4,7). A 20-24F chest tube is inserted via the uniport and anchored (Figure 10). The wound is then closed in layers around the chest tube. The skin must not be closed too tightly around the chest tube, otherwise skin ischemia and consequent poor wound healing can occur.
Post-operative care
A clinical pathway designed for uniportal patients is necessary to ensure that the patient receives the maximum benefit. It should incorporate all aspects of peri-operative management, including chest drainage, analgesia, mobilization, physiotherapy, investigations and communications with patient and family (17).
If a pre-incisional paravertebral blockade with bupivacaine bolus injection is given, further intercostal blockade or insertion of catheters for post-operative blockade infusion is unnecessary. In the author’s institutes, all patients after lobectomy receive regular oral paracetamol (acetaminophen) 1 g 6-hourly, supplemented by oral tramadol 50 mg 4–6-hourly only as required for breakthrough pain.
The sole chest drain is connected to a digital chest drainage system (Medela Thopaz, Medela AG, Baar, Switzerland) (18). A negative pressure of 15 cm H2O is applied initially, then reduced to gravity mode (8 cm H2O) on the morning after surgery. Drains are removed when the air flow is less than 40 mL/min for 6 hours with no ‘spikes’ of air leak during that time. Drainage volume and colour are nowadays not considered very important when determining when to remove chest drains.
Other lobes
Right middle lobe (RML)
The basic approach is mostly similar to that for the RUL. The RML is retracted laterally and posteriorly for most of the operation, allowing the hilar structures to be approached from an anterior position. Using an anterior-to-posterior unidirectional approach, the sequence of dissection would typically start with the pulmonary vein, followed by the lobar bronchus, pulmonary artery and fissure (oblique fissure often complete but horizontal fissure may require stapling). The challenge with the RML is that its hilum is closest to the uniport of all the lobes. That means that in smaller patients it is comparatively difficult to insert the entire head of the stapler to the hilar structures and to maneuver instruments inside.
Right lower lobe (RLL)
The RLL is retracted cephalad and laterally to expose the pulmonary ligament. Once this is divided, the inferior pulmonary vein is easily dissected and then divided. Rotation of the lung and reticulated stapler head clockwise (anteriorly) may help the emergence of the anvil if it is impacting onto the spine behind the vein. After the vein is divided, the RLL is retracted gently towards the feet, allowing completion of the oblique fissure between RLL and RML. This exposes the pulmonary artery in the fissure, which is dissected with sharp and blunt dissection (as described for the RUL) and then looped and staple-divided. Once the artery truncus is divided, retraction of the RLL laterally and posteriorly allows the RLL bronchus to be seen rising vertically from the bronchus intermedius up to the RLL. This is dissected and the stapler is inserted from a right-to-left direction to divide it.
Left upper lobe (LUL)
The same technique as with the RUL is used to dissect and divide the pulmonary vein. Once the vein is divided, the 1st and often 2nd pulmonary artery branches running vertically up to the LUL are seen behind. These can be dissected and stapled. However, these branches are often small and short and use of polymer ligating vascular clips are often very useful to avoid forcible insertion of a big stapler across them, which can cause tearing and avulsion. If the LUL is still retracted laterally, this LUL bronchus rises vertically on the monitor from the hilum to the LUL. The bronchus is dissected using Rumel forceps to dissect behind the bronchus. This step must be taken with extreme caution and the forceps must be kept in contact with the back wall of the bronchus at all times to avoid catastrophic damage to the interlobar pulmonary artery behind. Partial dissection or division of the anterior part of the interlobar fissure may often help improve exposure and access to the LUL bronchus. Once the bronchus is divided, lateral retraction of the LUL exposes any remaining pulmonary artery branches to the LUL behind where the bronchus used to be. These are variable in number and are dissected using sharp and blunt dissection in the same manner as previously described. They are then divided with a stapler or between polymer vascular ligating clips. These branches are very small and can be avulsed by rough dissection, so caution must be exercised.
Left lower lobe (LLL)
The LLL is retracted in a cephalad direction to expose the pulmonary ligament and then the pulmonary vein for dissection and staple-division. The LLL is then retracted towards the feet and the pulmonary artery dissected from within the fissure. Branch(es) to the LUL lingula are identified and preserved and the terminal branches to the LLL and LLL apical segment are dissected and divided with a stapler or polymer vascular ligating clips. Retraction of the LLL laterally and posteriorly allows the LLL bronchus to be seen rising vertically up to the LLL. This is dissected and staple-divided, taking care not to damage the interlobar pulmonary artery which runs behind and cephalad to the bronchus in this position. Sometimes, the interlobar fissure may be too fused and thick to allow dissection of the pulmonary artery from within the fissure. In this case, after division of the vein, the LLL is continued to be retracted in a cephalad direction. This exposes the LLL bronchus from a feet-to-head direction, and is dissected and divided next. After division of the bronchus, with the LLL kept in this retraction, the interlobar pulmonary artery is exposed from the same feet-to-head direction and its branches to the LLL and its superior segment can be dissected and divided. The lobectomy is then completed by staple-division of the fissure.
Comments
This article only outlines the technical aspects of performing uniportal VATS lobectomy. Results of performing this procedure can be found in other articles in this issue and elsewhere in the literature (5,6).
It must be acknowledged that at the time of this writing, there is very little clinical data showing a significant clinical advantage for uniportal VATS when performing lobectomy (19,20). However, there is sufficient evidence that the approach is safe (5,12-14,19,20). This means that VATS surgeons can safely learn and practice uniportal lobectomy, hopefully accumulating the clinical evidence that will ultimately show the real benefits of uniportal VATS in our specialty.
Acknowledgements
The author wishes to thank Dr. Beth Croce, Medical Illustrator for the Annals of Cardiothoracic Surgery, for her outstanding work in producing all the illustrations in this article to the specifications of the author.
Footnote
Conflicts of Interest: The author (ADLS) is a consultant for Medela AG (Baar, Switzerland).
References
- Sihoe AD, Yim AP. Video-assisted pulmonary resections. In: Pearson's Thoracic and Esophageal Surgery, Third Edition. Patterson GA, Cooper JD, Deslauriers J, et al. (Eds). Philadelphia: Elsevier.2008:970-88.
- Sihoe AD, Yim APC. VATS as a diagnostic tool. In: General Thoracic Surgery (7th Edition). Shields TW, Locicero J, Ponn RB, (Eds). Philadelphia:Lippincott Williams & Wilkins.2009:313-32.
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Sihoe AD. The Evolution of VATS Lobectomy. In: Cardoso P. eds. Topics in Thoracic Surgery. Rijeka: Intech, 2011:181-210.
- Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-17. [PubMed]
- Tan LJ, Sihoe AD, Liu LX, et al. (editors). Uniportal Video-Assisted Thoracic Surgery. Hong Kong: AME Publishing, 2015.
- Sihoe AD, Cheng LC. The Evolution of Minimally Invasive Surgery for Lung Cancer. HKMA CME Bulletin 2014:11-20.
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Sihoe AD, Chawla S, Paul S, et al. Technique for delivering large tumors in video-assisted thoracoscopic lobectomy. Asian Cardiovasc Thorac Ann 2014;22:319-28. [Crossref] [PubMed]
- Sihoe AD, Hiranandani R, Wong H, et al. Operating on a suspicious lung mass without a preoperative tissue diagnosis: pros and cons. Eur J Cardiothorac Surg 2013;44:231-7; discussion 237. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [Crossref] [PubMed]
- Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg 2007;31:203-8. [Crossref] [PubMed]
- Refai M, Brunelli A, Salati M, et al. Efficacy of anterior fissureless technique for right upper lobectomies: a case-matched analysis. Eur J Cardiothorac Surg 2011;39:1043-6. [Crossref] [PubMed]
- Sihoe AD, Yu PS, Kam TH, et al. Adherence to a Fast Track Clinical Pathway for Video Assisted Thoracoscopic Surgery: clinical importance and predictors. The 2015 Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothracic Surgery (ISMICS 2015), Germany, Berlin, 3-6 June 2015.
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiport video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015;20:813-9. [Crossref] [PubMed]





