Video assisted thoracic surgery (VATS) for recurrent thymoma
Introduction
Thymomas are the most common tumors of the anterior mediastinum. Radical surgical resection is the mainstay of treatment for this type of tumor and the most important prognostic factor (1-3). Unfortunately, in 8-30% of patients that undergo radical surgical resection, a recurrence of the tumor may occur over a wide range of time, from few months to several years after the first operation (4-7). The probability of recurrence seems to be related to the initial Masaoka stage of the disease (8,9), as well as the WHO histology (10) with an increased relapse rate for Masaoka stage III and types B2-3 primary tumors. The most common site of tumour relapse from thymoma is the thoracic cavity, mainly in the form of single or multiple pleuro-pericardial implants (46-80% of all recurrences, probably due to the seeding of the pleural cavity during dissection of the tumor or spillage from the surface of the invaded capsule) (3,4,11,12). In a minority of patients, the relapse may localize in the anterior mediastinum due to incomplete resection or contamination of the field from extracapsular invasion. The management of thymoma recurrence still remains unclear and the role of surgery is not yet well defined in terms of indication, surgical access and extent of resection.
Evidence supporting surgery for recurrent thymoma
Treatment for recurrence may not be easy and the optimal strategy for managing these patients is still a matter of debate. Thymoma is a rare disease and recurrences are even rarer; therefore, to date, no randomized clinical trials have been performed to explore the best management of this disease. The majority of available data comes from retrospective single or multiinstitutional series (the largest of which includes 103 patients) (2,4-10,13-20), that have analyzed the results of surgical approach to thymoma recurrence and compared these with non-surgical treatments (i.e., chemotherapy, radiotherapy or a combination of both). Various authors (4-9,13-19) have emphasized the efficacy of surgical re-resection to prolong the survival of these patients; other authors (10,20) found no differences in the results obtained through surgery and chemo- or radiotherapy. However, the results of these studies are often burdened by significant biases, related to the heterogeneous degrees of severity, variable patterns of recurrence, different types of therapeutic approach and various selection criteria (regarding the presence of primary Masaoka stage IVa, type C thymic carcinoma, recurrence on patients with incomplete resection, and technical resectability). In fact, the comparison of patients treated by surgery with those receiving chemo-radiotherapy is often inappropriate due to differences in extension (single or multiple relapses), location of the disease (local vs. distant metastases) and performance status of the patients. Currently, the general consensus indicates surgical resection of cases of a single, potentially resectable, loco-regional thymoma recurrence, irrespective of location (i.e., pleural, pulmonary or mediastinal) if the relapse is judged resectable. This approach is associated with a good long-term prognosis (13,14,17). In 2008, Davenport et al. (21) published a systematic review targeted to provide some evidence-based recommendations about the role of surgery in the management of primary and recurrent thymomas. The authors concluded that surgical treatment of relapses seems acceptable, although the data supporting such a recommendation is methodologically weak and based only on reports coming from retrospective series. In a meta-analysis recently published by Hamaji et al. (8) considering 11 studies, the authors reached similar conclusions, underlining that the best results may be obtained when a complete resection is anticipated by the preoperative radiological assessment. It is undoubted, however, that given most of the studies evaluating the role of surgery for recurrent thymoma are retrospective, they suffer from an inherent selection bias. Surgery is usually reserved for patients with limited disease (the best prognostic factor) and better performance status, with a theoretical predicted survival advantage. A survey among the European Society of Thoracic Surgeons (ESTS) members (22) published in 2011, reported a general agreement regarding the preference (91%) for surgical approach to recurrence when resection is feasible. In this survey, some centres reported that they performed multiple subsequent resections in patients with repeated recurrence. Furthermore, many centres added in their comments that correct patient selection is crucial and that they proceed to resection only when complete resection may be anticipated. The average rates of 5- and 10-year overall survival after recurrence are 70.9%±16.2% (range, 40-85.7%) and 49.6%±27.4%, respectively for surgical series.
Type of surgery
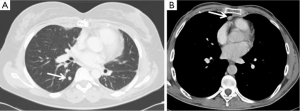
Even for recurrence, the radicality of re-resection is a major prognostic factor. Therefore, a complete macroscopic resection represents the goal and the surgical approach (both in term of way of approach and extension of resection) should be planned and tailored to accomplish this aim. The number and location of recurrence are determinant in the surgical planning: a single pulmonary recurrence may be easily treated by a wedge (if peripheral) or anatomic (if central) pulmonary resection (Figure 1A). Similarly, a mediastinal non-invasive recurrence, particularly if characterized by unilateral predominance may be approached from the right or left chest cavity by thoracoscopic or robotic approach or thoracotomy, avoiding a sometimes difficult redo-sternotomy (Figure 1B).
A more complex discussion is required when there is evidence of single (Figure 2A,B) or multiple (Figure 2C) pleuro-pericardial implants. In these cases, the majority of the authors (4-6,14,15,17,18) advocate a limited pleural resection or a partial pleurectomy, comprising only the lesions macroscopically evident, in case of single or limited pleural relapses.
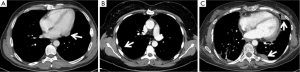
Some authors, however, have described extended resections, from pleurectomy/decortications to extrapleural pneumonectomy. In some cases, these procedures are accompanied by induction chemotherapy or intraoperative hyperthermic pleural cavity irrigation or chemoperfusion, both for Masaoka stage IVa or diffuse pleural relapses. The results of these studies are controversial and often poor (23-25).
Role of minimally invasive approach
Looking at the available literature (Table 1), the most common surgical approach for thymoma recurrences is represented by thoracotomy. The video assisted thoracic surgery (VATS) approach is rarely reported, and mainly reserved for wedge resection of pulmonary nodules or single pleural relapse (17,19,27). There are multiple reasons for this low rate of minimally invasive approach, including: (I) the majority of the series covers a long period of time and a large number of cases have been treated in the “pre-VATS era” or in a period of low adoption of VATS; (II) in a certain percentage of cases, a VATS approach may be contraindicated for technical reasons (e.g., diffuse recurrence, previous thoracotomy, adhesions, previous irradiation); (III) a total pleurectomy or pleurectomy/decortication by VATS in case of diffuse pleural recurrence (the most common type of relapse) is considered difficult and often inadequate to obtain a macroscopic radical resection.
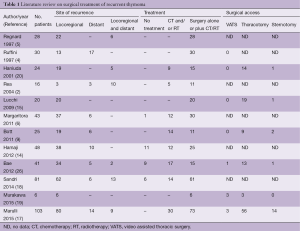
Full table
VATS for recurrent thymoma: pros & cons
Given the widespread adoption of the VATS approach in the last decade for a variety of thoracic malignant diseases (primary and metastatic tumors of the chest cavity), this approach merits some consideration regarding its potential application for recurrent thymoma.
Firstly, recent technical advancements (i.e., dedicated instruments, high definition optics, new devices) have increased the quality and the range of operations that may be safely performed by VATS. Second, the well-known advantages of minimally invasive surgery (limited surgical trauma, low complications, short hospitalization, better cosmetic results, and early recovery of pulmonary function) may have a particular positive clinical impact in this subset of patients, often affected by myasthenia gravis. Third, the high definition of modern preoperative diagnostic radiological techniques allows for a precise assessment of the extent of the recurrent disease and a reliable prediction of the surgical resectability. In this way, it is possible to plan the best approach and the extension of surgical resection.
It is well established that the minimally invasive approach cannot threaten the quality of operation in term of radicality of resection. This represents the most important prognostic factor. To date, single pleuro-pulmonary or mediastinal unilateral non-invasive recurrences represent the best indication for VATS resection, but the role of minimally invasive approach for more extended operations (i.e., partial or extended pleurectomy) needs to be clarified.
Conclusions
In conclusion, surgery for recurrent thymoma is effective and safe, leading to good long-term survival rates. Complete macroscopic resection is the goal also for recurrent disease and should be accomplished, if possible. In our opinion, the role of VATS for recurrent thymoma is still unclear and not well explored. It is very likely that over the following years, an increasing amount of research will be available aiming at a more precise and established definition of the indications for minimally invasive approaches in thymic relapses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [PubMed]
- Regnard JF, Zinzindohoue F, Magdeleinat P, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8. [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Single-centre 40-year results of redo operation for recurrent thymomas. Eur J Cardiothorac Surg 2011;40:894-900. [PubMed]
- Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985;39:165-9. [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of surgical versus nonsurgical management of recurrent thymoma. Ann Thorac Surg 2014;98:748-55. [PubMed]
- Bott MJ, Wang H, Travis W, et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg 2011;92:1984-91; discussion 1991-2.
- Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40-4. [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26-31. [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Number of recurrent lesions is a prognostic factor in recurrent thymoma. Interact Cardiovasc Thorac Surg 2011;13:21-4. [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [PubMed]
- Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185-9. [PubMed]
- Urgesi A, Monetti U, Rossi G, et al. Aggressive treatment of intrathoracic recurrences of thymoma. Radiother Oncol 1992;24:221-5. [PubMed]
- Marulli G, Margaritora S, Lucchi M, et al. Surgical treatment of recurrent thymoma: is it worthwhile?† Eur J Cardiothorac Surg 2015. [Epub ahead of print]. [PubMed]
- Sandri A, Cusumano G, Lococo F, et al. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol 2014;9:1796-804. [PubMed]
- Murakawa T, Karasaki T, Kitano K, et al. Invasive thymoma disseminated into the pleural cavity: mid-term results of surgical resection. Eur J Cardiothorac Surg 2015;47:567-72. [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [PubMed]
- Ruffini E, Van Raemdonck D, Detterbeck F, et al. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol 2011;6:614-23. [PubMed]
- Yellin A, Simansky DA, Ben-Avi R, et al. Resection and heated pleural chemoperfusion in patients with thymic epithelial malignant disease and pleural spread: a single-institution experience. J Thorac Cardiovasc Surg 2013;145:83-7; discussion 87-9. [PubMed]
- Fabre D, Fadel E, Mussot S, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardiothorac Surg 2011;39:e133-8. [PubMed]
- Belcher E, Hardwick T, Lal R, et al. Induction chemotherapy, cytoreductive surgery and intraoperative hyperthermic pleural irrigation in patients with stage IVA thymoma. Interact Cardiovasc Thorac Surg 2011;12:744-7. [PubMed]
- Bae MK, Byun CS, Lee CY, et al. Clinical outcomes and prognosis of recurrent thymoma management. J Thorac Oncol 2012;7:1304-14. [PubMed]
- Heyman SR, De Raeve H, Mercelis R, et al. Recurrent myasthenia gravis due to a pleural implant 3 years after radical thymectomy. Ann Thorac Surg 2008;86:299-301. [PubMed]





