Troubleshooting thoracoscopic anterior mediastinal surgery: lessons learned from thoracoscopic lobectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) lobectomy for lung cancer has less morbidity and at least equivalent oncologic results compared to open approaches (1,2). Several single and multi-center studies have also demonstrated the technical feasibility of VATS for mediastinal surgery, with outcomes comparable to conventional open procedures (3,4). Although robotic-assisted resection of mediastinal masses is also promising and effective, robot use can be limited by cost and equipment availability (5). Hence, while thoracic surgeons should develop the ability to perform VATS resection of mediastinal masses, gaining experience in these procedures can be limited by the relative rarity of mediastinal pathology compared to lung cancer. However, lessons learned from experience with VATS lobectomy can be applied to the mediastinum to speed up the learning curve.
Through this troubleshooting guide, we sought to compile ‘tips’ for managing the numerous intra-operative technical difficulties that can arise during thoracoscopic anterior mediastinal procedures.
Patient and methods
General operative considerations
The preoperative patient evaluation for VATS anterior mediastinal surgery is generally similar to that for pulmonary resections (6). Obtaining computed tomography (CT) scans with intravenous contrast is particularly important to evaluate the relationship of the mass to other mediastinal structures, to evaluate resectability and to plan approach. Because 20-25% of thymoma patients have myasthenia gravis (MG), patients with known or suspected thymoma should be screened for MG prior to resection to aid in perioperative management (7). Induction therapy is used for germ cell tumors and can be considered for marginally resectable tumors such as Stage III thymomas or thymic carcinomas, though, surgeons must subsequently be cognizant of the potentially higher risk of intraoperative issues (such as bleeding) and postoperative complications (including pulmonary insufficiency) (7,8).
Thoracoscopic mediastinal approaches include unilateral VATS, bilateral VATS, or the substernal approach (9). If access to the contralateral chest to aid in exposure or dissection may be necessary, surgeons should advocate for a double-lumen endotracheal tube rather than a bronchial blocker to achieve single-lung isolation. Intravenous access in the groin or lower extremity should also be obtained if significant manipulation of the innominate vein or superior vena cava is considered possible. Insufflation with carbon dioxide (CO2) (4-8 mmHg) may improve exposure, but CO2 retention must be monitored (6). Standard post-operative management including adequate pain control, pulmonary toilet, and early ambulation is also pivotal in minimizing complications.
Patient positioning
Generally, patients are placed in the supine position with one or both arms extended, although a particular side may be preferentially exposed to optimize lateral access. The ipsilateral upper extremity is positioned to prevent traction injury at the shoulder. The operative field must be sterilized widely, to allow multiple port placements and allow conversion to bilateral VATS, anterior thoracotomy, or median sternotomy if concerns regarding safety or complete resection arise intraoperatively.
Troubleshooting guide
This troubleshooting guide focuses on technical challenges that can occur during VATS anterior mediastinal procedures, particularly VATS thymectomy (Table 1). The following sections offer maneuvers to manage these intra-operative technical difficulties, based on lessons learned from VATS lobectomy experience.
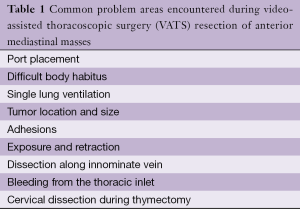
Full table
Port placement
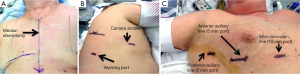
Ports placement for thoracoscopic mediastinal resection with the patient in the supine position differs from port placement when resection is performed in the lateral position. With the patient supine or slightly turned, ports are placed in the 5th or 6th intercostal space (in the breast crease) in the mid-clavicular line (10 mm), the anterior axillary line (5 mm), and the posterior axillary line in the axilla (5 mm) (Figure 1). The most anterior port should take advantage of the larger intercostal space to facilitate subsequent specimen removal without requiring significant incision enlargement. Although the more lateral and posterior incisions may be more difficult to place depending on the habitus and component of adipose tissue, all ports should be in the same intercostal space if possible to minimize pain. If the bilateral approach is used, one or two contralateral 5 mm incisions may be added if extended visualization is needed.
Difficult body habitus
Obtaining access for mediastinal dissection can be difficult in patients with significant subcutaneous obesity or mediastinal adipose tissue. For subcutaneous obesity, longer ports (up to 10 cm) can provide easier access into the chest. A wound protector may also adequately retract subcutaneous tissue in the access incision if the approach is not completely portal. Retracting and fixating the breasts prior to sterile preparation may be necessary in obese females, which can be challenging when planning a bilateral approach. Patients with significant abdominal obesity may require more superior port placement or moderate reverse Trendelenberg positioning to lessen the effect of high diaphragms.
Access for left thoracoscopic mediastinal surgery may be compromised by the smaller operative thoracic space. Full lung deflation should occur prior to dissection, and contralateral tidal volumes should be minimized. Rolling the operating table can also allow gravity to improve lung retraction. Particular attention must be paid to port placement in patients with cardiomegaly, for whom a right-sided VATS may be preferable if feasible.
Single lung ventilation
Single lung ventilation with the lowest possible concentration of inspired oxygen should be achieved to minimize the risk of postoperative pulmonary toxicities, especially in patients who had induction therapy (8). Adequate lung deflation facilitates every thoracoscopic procedure, although probably less important for these resections. Physiologically slow development of atelectasis due to chronic obstructive lung disease can cause inadequate deflation, and the most common etiology for relative hypoxia is slow hypoxic pulmonary vasoconstriction. A little time and patience will correct both conditions (8).
If hypoxia or incomplete atelectasis persists, correct positioning of the endotracheal tube and adequate inflation of the tracheal and bronchial balloons, without the bronchial balloon obstructing the contralateral bronchus, should be confirmed. Endotracheal suctioning should help clear retained secretions. Additionally, the tube should not be advanced too far into the left main bronchus so that only the lower lobe is ventilated. Pressure controlled ventilation that minimizes tidal volume (<300 mL) should be employed. Adequate muscle relaxation will optimize thoracic capacity and eliminate spontaneous ventilation. Suction catheters are often used to continuously aspirate the ipsilateral bronchus, but probably have no significant effect. If the above maneuvers are not adequate, CO2 insufflation may be utilized if the port incisions have not been extended.
Tumor size and location
Large tumors close to the thoracic inlet reduce the space available to manipulate, retract and dissect the tumor, while protecting the phrenic nerves, recurrent laryngeal nerves and the vasculature. A unilateral thoracoscopic approach in the supine position is facilitated if there is a sidedness to anterior tumors. Bilateral thoracoscopic access can improve visualization to protect the phrenic nerve and facilitate dissection around difficult areas such as the innominate vein, superior vena cava, and the aorto-pulmonary window. Dissection of the lower mediastinum is easier than upper mediastinum for both anterior and posterior tumors, although larger tumors (>7 cm) in any location are challenging and may be best approached via median sternotomy. Induction chemotherapy may accomplish significant downsizing of Masoaka stage III thymomas (8), but persistent adherence or unrecognized invasion into vascular structures may pose particular challenges.
Additionally, mediastinal tumors (especially upper mediastinal tumors) may represent the best application for robotics in thoracic surgery. Minimizing tidal volumes to the contralateral lung also improves the ipsilateral thoracic volume. Sternal retraction may incrementally increase the working space in the thoracic inlet to prevent the need to convert to an open procedure. The sternum may be retracted upward by inserting a standard sternal retractor, conventionally used during internal thoracic artery dissection, through a small incision at the suprasternal notch. Finally, judicious retraction of the pericardium with a thoracoscopic instrument, while carefully avoiding systemic hypotension, can greatly facilitate substernal access.
Adhesions
The presence of significant adhesions is not as common as for pulmonary surgery overall, but previous median sternotomy is problematic. Although uncommon, surgeons must identify and be aware of patent grafts in patients with previous coronary artery bypass grafting. The access incision should be made first when adhesions are anticipated, allowing uniportal exposure and adhesiolysis. Only 1 cm of space is needed to begin adhesiolysis, and the 2nd port is added when the lateral dissection allows safe placement. The use of an extended electrocautery is essential, and other energy sources are sometimes helpful.
Exposure and retraction
Achieving adequate retraction for thoracoscopic mediastinal resection is more important than for pulmonary surgery. More than one retracting instrument is rarely required but exposure may still be limited during thymectomy, especially with a left-sided approach. Several maneuvers may improve exposure. Grasping and posterior retraction will improve exposure during thymectomy, but hemodynamics must be monitored to avoid excessive limitation of venous cardiac inflow. CO2 insufflation, sternal elevation, and opening the contralateral pleura may all improve exposure.
Dissection along the innominate vein
Dissection along the inferior aspect of the innominate vein is undertaken after confirming the absence of invasion by the tumor or process being resected. Either left to right or right to left dissection can be performed, with the direction usually determined by port placement. The use of an energy source is essential to manage thymic veins, with clips used as needed on the innominate vein side. While dissection is usually straightforward, the surgeon should always be prepared for control of hemorrhage and conversion. Sometimes, the area just posterior to the innominate vein is the most difficult dissection. Residual thymic tissue should not be left in this area, whether operating for malignancy or for MG.
Bleeding from the thoracic inlet
As stated above, the surgeon should always be prepared. It is crucial that the surgeon does not panic and has prepared the operative team for managing bleeding. The most important source of bleeding during dissection in the thoracic inlet is from the innominate vein, and the surgeon should not attempt to clamp the vein unless the injury is discreet and well-visualized to avoid extending the injury. Unlike bleeding from the pulmonary artery, bleeding from the innominate vein will not resolve with controlled pressure.
A long curved sponge stick should be used to tamponade and control the bleeding site, but conversion may be necessary. Bleeding from the right internal mammary vein can be managed thoracoscopically with a clip, energy device, or suture.
Cervical dissection during thymectomy
In some patients, the above maneuvers are not successful in exposing the thoracic inlet for dissection of the thymic cervical horns during thymectomy for MG. In this situation, a standard cervical counter incision may be made (extending the incision in the sternal notch if a sternal retractor was utilized). This incision allows complete exposure of the cervical thymus and is likely better tolerated than conversion to sternotomy.
Results
The lessons learned from an extensive VATS lobectomy experience have allowed the authors to apply the VATS approach to other challenging resections (2,6). This troubleshooting guide identifies the most common issues that may complicate VATS anterior mediastinal surgery, and provides guidance in management with a goal of minimizing the need for open approaches. Although conversion to an open approach is sometimes necessary for various reasons, integrating some of these troubleshooting ‘tips’ in clinical practice could help circumvent some of the anticipated technical challenges.
Comments
VATS resection of mediastinal masses may be less complex than VATS pulmonary or esophageal resections, but has a unique set of challenges. Although conversion to a conventional approach should always be performed if required for ensuring safety or complete resection, avoidance of conversion improves outcomes. A clear pre-operative plan (including a plan for conversion), training of the entire operating team regarding the principles of minimally invasive surgery, knowledge of situations most likely to be problematic, and the ability to avoid, recognize, and manage intraoperative complications is required to optimize outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- D'Amico TA. Long-Term Outcomes of Thoracoscopic Lobectomy. Thorac Surg Clin 2008;18:259-62. [PubMed]
- Demmy TL, Krasna MJ, Detterbeck FC, et al. Multicenter VATS experience with mediastinal tumors. Ann Thorac Surg 1998;66:187-92. [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 1753.
- Lucchi M, Ricciardi R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg 2009;35:812-6. [PubMed]
- Grichnik KP, D'Amico TA. Acute Lung Injury and Acute Respiratory Distress Syndrome After Pulmonary Resection. Seminars in Cardiothoracic & Vascular Anesthesia 2004;8:317-34. [PubMed]
- Shigemura N, Yim AP. Variation in the approach to VATS lobectomy: effect on the evaluation of surgical morbidity following VATS lobectomy for the treatment of stage I non-small cell lung cancer. Thorac Surg Clin 2007;17:233-9. ix. [PubMed]





