Minimally invasive thymectomy: the Mayo Clinic experience
Introduction
Thymectomy is an acceptable therapy in the comprehensive care of myasthenia gravis (MG) and in undetermined lesions (not thought to be lymphoma) that are found within the anterior mediastinum by cross-sectional imaging (1-3). Primary epithelial thymic tumors are discovered in approximately 50% of all anterior mediastinal masses, of which thymoma is the most common (4,5). The efficacy of surgery in managing thymic diseases, including the ability to improve symptoms of inadequately controlled myasthenia, is contingent upon complete excision of all thymic and perithymic adipose tissue (6).
Video-assisted thoracoscopic surgery (VATS) and the da Vinci robotic system (Intuitive Surgical Inc., Sunnyvale, CA, USA) offer a minimally invasive approach to thymectomy with potentially less morbidity. However, controversy exists regarding the appropriateness of minimally invasive thymectomy (MIT) when employed for surgical resection of thymoma and other malignant neoplasms. Although evidence substantiating MIT as an effective treatment with less operative trauma, shorter length of hospital stay, fewer pulmonary complications and more satisfactory cosmetic results without compromising surgical outcomes is available, few studies have compared the two MIT approaches (7,8). The primary objective of the present study is to analyze patient and surgical outcomes in our experience with VATS and robotic-assisted thymectomy.
Methods
All patients who underwent resection for a thymic lesion at our institution between January 1, 1995 and February 28, 2015 were identified from a prospectively maintained surgical database. The medical records were retrospectively reviewed for demographic data, presenting symptoms, operative procedures, complications, pathology, recurrence, and date of last follow-up or death. The revised Masaoka staging system was used for staging thymomatous neoplasms (9,10). Histologic type was classified according to the 2004 revision of the World Health Organization (WHO) classification of thymic epithelial tumors (11).
The Mayo Foundation Institutional Review Board approved the current study with waiver of informed consent. During the study period, 510 thymectomies were performed at Mayo Clinic Rochester. Of the 510 thymus glands removed, 56 (11%) were performed using a minimally invasive approach: 45 (80.4%) had a VATS resection whereas 11 (19.6%) had a robotic-assisted resection. There were 25 men (44.6%) and 31 women (55.4%) with a median age of 55 years (range, 23-87 years) at the initial thymectomy.
Descriptive statistics for categorical variables are reported as frequency and percentage; continuous variables are reported as mean (SD) or median (range) as appropriate. Patient characteristics in the VATS and robotic resection groups were compared using Fisher’s exact test for categorical variables and the Wilcoxon test for continuous variables. A P value of less than 0.05 was considered statistically significant. The SAS software (JMP, Version 10., SAS Inc., Cary, NC, 1987-2007, USA) was used for statistical analysis.
Surgical technique
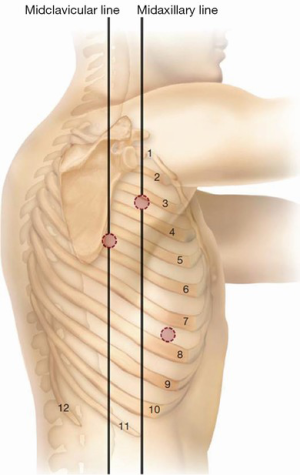
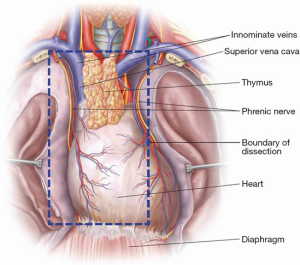
The right-sided approach to VATS thymectomy is often preferred given the physical constraint from the heart on the left. However, VATS thymectomy can be safely performed with solitary entry into either pleural space. Herein we present our technique of right-sided VATS thymectomy. The patient is positioned in the left lateral decubitus position supported with axillary roll and bean bag when necessary. The patient’s anterior superior iliac spine should be positioned at the break in the bed with the bed flexed to at least 30 degrees to open the intercostal rib spaces, facilitate port positioning, and allow the lungs to fall away posteriorly. We prefer general anesthesia with a double-lumen endotracheal tube with confirmation of position via bronchoscopy. The right arm is positioned over the left arm and supported by a brace. All pressure points are padded. With a pause in ventilation, the first 5 mm port is placed between the 5th or 6th intercostal space (ICS) along the posterior axillary line (Figure 1). Once entry into the pleural space is confirmed with finger sweep, the thoracoscope is inserted to inspect the pleural cavity for any pathology (effusions or suspicious lesions). Any suspicious lesions are routinely biopsied. Under direct vision, a second 5 mm port is inserted at the 3rd or 4th ICS along the mid-axillary line (Figure 1). This will serve as the camera port for the remainder of dissection. The third port is then placed at the 5th or 6th ICS along the anterior axillary line (Figure 1). Port location can be varied according to surgeon preference and additional cannulae may be placed if visualization or dissection is not optimal. The boundaries of surgical resection should include all thymic and perithymic tissue between the phrenic nerves and from the innominate vein superiorly to the diaphragm inferiorly (Figure 2). The phrenic nerve should be identified prior to proceeding, with division of the right inferior pole of the thymus from the pericardial fat pad using an advanced vessel sealing device. With electrocautery on a low setting, the mediastinal pleura can be scored 1-2 cm medial to the phrenic nerve to facilitate lateral dissection as the surgeon proceeds cranially to the right superior pole. Medium to large-sized venous branches from the innominate vein may be encountered and are effectively controlled with clip placement followed by sharp or electrocautery division. A vessel sealing device is used to divide the attachments of the thyrothymic ligament. Dissection is carried to the contralateral side beyond the midline dividing superior and inferior attachments in similar fashion. Protecting the contralateral phrenic nerve and vascular bundle remains an essential step. If visualization of these structures is impaired, patient repositioning with contralateral VATS should be undertaken to avoid inadvertent injury and subsequent hemidiaphragm paralysis. The specimen is placed in a bag and removed through a protected port. The port incision may need to be lengthened for larger sized glands or neoplasms to allow extraction of the specimen. A small bore chest tube may be placed using the skin incision from the third port and placed to −20 cmH2O suction. The intercostal muscle layers and skin are then closed.
Results
Demographic data
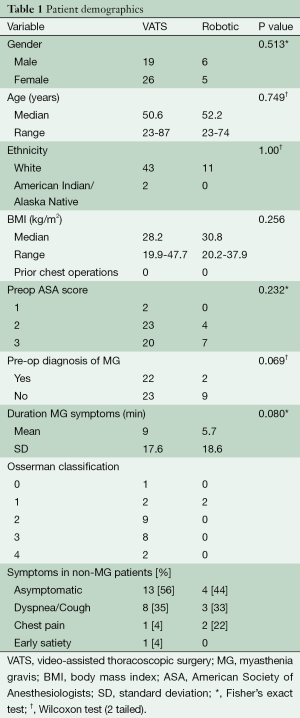
The patients’ age, sex, functional status, history of MG, and presenting symptoms in both MG and non-MG at the time of thymectomy are shown in Table 1. There were no significant differences among patients selected for either VATS or robotic-assisted thymectomy.
Full table
A slight female preponderance (55.4%) existed in our cohort, with patients most commonly presenting in the fifth decade of life. Over 96% of patients were ethnically white and only 15 (27%) patients had a normal BMI (≥18.5 to 24.9 kg/m2) at the time of operation. Nineteen (34%) were overweight (≥25.0 to 29.9 kg/m2), and 23 (41%) were obese (≥30 kg/m2) according to the National Institute of Health (NIH) and WHO classification of obesity (12,13).
MG was preoperatively diagnosed in 24 (42.9%) patients, all of who were seropositive for acetylcholine receptor antibodies and exhibited classic findings on electromyographic studies. Only one patient with MG was asymptomatic (Modified Osserman Stage 0) and was counseled to undergo thymectomy due to an enlarging thymic nodule on serial imaging with computed tomography (CT). All other MG patients underwent thymectomy for symptom improvement. The majority of MG patients 17 (71%) were either a Modified Osserman Stage 2 or 3 pre-operatively.
The most common presentation of non-MG patients with thymic lesions requiring resection was an incidentally discovered asymptomatic anterior mediastinal mass by CT imaging, found in 17 (53.1%) patients. Cough or dyspnea was observed in 11 (34%), and chest pain in three (9%) patients.
Perioperative outcomes
Pathologic analyses of the 56 resected thymic glands are shown in Table 2. A total of 32 tumors with six different histologic features were identified. Twenty-four thymic glands were benign with 11 (19.6%) containing hyperplastic tissue. The most common tumor was a thymoma in 27 (84.3%) patients harboring a neoplasm. An encapsulated, non-invading, stage I thymoma was identified in 18 (67%) pathologic specimens whereas the remainder, nine (33%), were stage IIA exhibiting capsular invasion. All thymomas underwent an R0 resection with negative surgical margins. All patients with Stage IIA tumors were evaluated post-operatively by medical oncology with no adjuvant treatment recommended. The most common histologic thymoma, according the WHO classification, in our series was type AB (mixed) in 11 (41%) patients.

Full table
The mean size of all thymic glands removed was 17.1 cm × 7.5 cm × 2.4 cm, whereas the mean tumor size was 2.8 cm × 2.0 cm ×1.4 cm with a mean overall thymic gland weight of 69.9 (range, 30-330) mg. The thymus or tumor within it was not significantly different in size among those resected with either VATS or robotic approaches (P=0.9 and P=0.3, respectively).
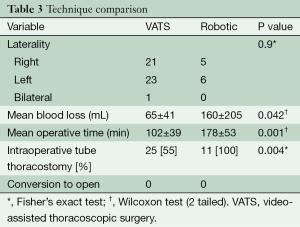
Total thymectomy by VATS was performed in 45 (80.4%) patients, whereas robotic-assisted resections were performed in 11 (19.6%). Surgical technique was chosen based on surgeon preference and technical ability. Operative comparison is summarized in Table 3. VATS thymectomy had significantly lower blood loss (65±41 mL, range, 20-250 mL) and operative time (102±39 min, range, 48-231 min) than the blood loss (160±205 mL, range, 50-750 mL, P=0.042) and operative time (178±53 min, range, 114-258 min, P=0.011) of robotic-assisted thymectomy. In addition, intraoperative tube thoracostomy was performed less frequently in VATS procedures 25 (55%) than in robotic 11 (100%) (P=0.004). Laterality in surgical approach was not significantly different. Right-sided VATS and robotic procedures occurred in 21/45 (47%) and 5/11 (45%) respectively. Left-sided VATS and robotic procedures occurred in 23/45 (51%) and 6/11 (55%) respectively (P=0.9). Only one VATS case required bilateral cannulae to adequately visualize the contralateral phrenic nerve to prevent injury. There were no open conversions with either MIT approach.
Full table
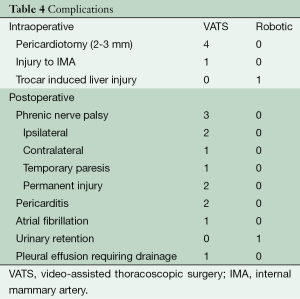
No mortality occurred in either group. Intra-operative and post-operative morbidity is reported in Table 4. Intraoperative morbidity occurred in 6/56 (11%) patients, the most common being a small (2-3 mm) pericardiotomy in four VATS patients, which were all left alone. Trocar-induced liver injury during a robotic resection required urgent laparoscopic exploration and hemorrhage control (no transfusions were required). Overall post-operative morbidity occurred in 8/56 (14%) patients. VATS complications 7/45 (16%), included phrenic nerve palsy in three (7%), pericarditis in two (4%), atrial fibrillation in one (2%), and a pleural effusion requiring catheter drainage in one (2%). Phrenic nerve palsy was ipsilateral in 2/3 and contralateral in 1/3. Two patients did not have resolution of phrenic nerve palsy: one required sural to phrenic nerve grafting 9 months postoperatively (which was unsuccessful), and the other continued to have paradoxical hemidiaphragm motion on fluoroscopic sniff testing 7 months postoperatively. A single robotic patient 1/11 (9%) developed urinary retention requiring home going catheterization. Mean length of stay was not significantly different: VATS (1.5 days, range, 1-4 days) vs. robotic (2.1 days, range, 1-5 days, P=0.05).
Full table
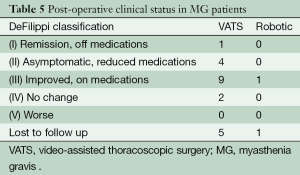
Clinical improvement, according to the DeFelippi classification, in the 23/24 (symptomatic) MG patients is reported in Table 5. Symptom improvement with reduced immunosuppressive medication was observed in 14/16 (88%) VATS patients and 1/2 (50%) robotic patients with a mean follow-up of 18.4 (range, 1-79) months. The cumulative complete symptom remission rate in VATS patients was 5% vs. 0% in the robotically resected patients. A total of six MG patients were lost to follow-up (five VATS, one robotic), thus accurate documentation of clinical improvement is lacking.
Full table
Discussion
The aim of this study was to review our experience with MIT and compare operative and clinical outcomes between VATS and robotic-assisted techniques. We found that total thymectomy can be safely performed with either surgical approach with minimal morbidity without mortality. Compared with robotic-assisted thymectomy, the VATS approach offered a surgical advantage in terms of significantly less operative time (102±39 min) and blood loss (65±41 mL). Although length of stay was not significantly different between the two MIT approaches in our series, VATS patients were discharged earlier. Interestingly, the one case of urinary retention in our series was in a patient who underwent robotic resection with prolonged operative time (232 min) and subsequent increased length of stay (3 days) due to the need for in and out catheterization and required teaching. Other investigators have identified a similar trend in swifter operative times with VATS thymectomy. Ye (14) reported median VATS time of 170 min in a series of 125 VATS thymectomies, whereas Li and Wang (15) reported 105 min among 43 thoracoscopic cases. However, a recent study by Rückert et al. (16) reported the opposite trend with robotic cases performed with greater surgical efficiency (187 vs. 200 min). In that series, the cumulative complete remission rate of MG for robotic thymectomy was also significantly better (39% vs. 20%) than VATS with 42 months follow-up. While the most surgically efficient approach to MIT remains controversial, it is agreed that thymectomy must be complete, especially when employed for MG or primary resection of malignant neoplasms (i.e., thymoma, thymic carcinoma, thymic carcinoid). A study by Hamaji et al. (17) emphasizes the importance of complete thymectomy at the time of initial operation for thymoma as recurrence, due to incomplete resection, had a significantly adverse effect on overall survival. In addition, on multivariate analysis, only surgical management was associated with prolonged survival and improved progression-free interval in recurrent thymoma when compared with chemoradiotherapy. Contrary to the better survival outcomes among surgically managed recurrent thymoma, recurrent thymic or carcinoid tumors do not have improved survival with surgical therapy.
Surgical technique, whether VATS or robotic, was not influenced by gland size. All patients in our series had preoperative CT imaging and there were no difference noted in gland size or weight among pathologic specimens with either VATS or robotic-assisted resection. The decision regarding which side of the chest to enter and the number of cannulae to insert for MIT depended upon preoperative imaging and surgeon experience. In our series, three-port VATS and robotic techniques were employed. In 2004, Rocco and associates (18,19) described a uniport thoracoscopic approach to limited pulmonary resections. Since then, uniport VATS has been employed for a variety of thoracic diseases including thymectomy (20).
Our findings of clinical improvement among MG patients following thymectomy support the findings of other researchers. At mean follow-up of 18 months, we report that 88% of VATS and 50% of robotically resected patients enjoyed symptom improvement with reduced immunosuppressive medication. Unfortunately, 74% of patients in our series reside out of state, limiting our long-term follow-up. A recent meta-analysis of 28 controlled studies showed that MG patients undergoing thymectomy were twice as likely to attain medication-free remission, 1.6 times more likely to become asymptomatic, and 1.7 times more likely to clinically improve (21). Similar findings have been noted for patients undergoing VATS and robotic thymectomy (22-29).
Our results need to be interpreted in the context of several limitations. This was a single-institution experience, and thus the issue of external validity is relevant. Our data set might also not be readily applicable to other patients with thymic diseases. Although we analyzed all patients treated with MIT over a 20-year period, there may be some degree of selection bias among MIT patients, as evidenced by only early stage thymomas discovered on pathologic specimens with relatively bland histologic features by WHO classification. Furthermore, the high rate of complete (R0) surgical resection with no recurrences identified at the time of follow-up, raises the possibility that MIT patients had less extensive disease and would expect to have better outcomes as the incidence of recurrent thymic tumors typically ranges from 10% to 30% and is related to initial stage of disease as well as histologic features (WHO classification) (9,30-35). Finally, the duration of follow-up was relatively limited given the majority of patients in our series live out of state.
In conclusion, we believe that MIT can be performed for both non-neoplastic and neoplastic thymic diseases with minimal morbidity and mortality. While we are gaining experience with the da Vinci robotic system (Intuitive Surgical, Inc., USA), we still more commonly perform VATS thymectomy at our institution. Our data suggest that we currently perform VATS thymectomy with greater surgical efficiency, less blood loss, less need for tube thoracostomy, and are able to discharge patients slightly earlier than robotic-assisted procedures. It has been estimated that 50 identical robotic cases are required to perform any specific robotic surgery with consistent operative time and predictable outcome (36). With this learning curve in mind, we believe consideration of MIT should be pursued for all symptomatic MG patients with inadequate medical treatment and for all locoregional thymic neoplasms in patients who can tolerate single lung ventilation and in whom complete resection appears feasible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blalock A HA, Ford FF, Lilienthal J Jr. The treatment of myasthenia gravis by removal of the thymus gland. Br J Surg 1946;32:201-14.
- Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997;226:324-34; discussion 334-5. [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [PubMed]
- Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22. [PubMed]
- Rosenow EC 3rd, Hurley BT. Disorders of the Thymus. A Review. Arch Intern Med 1984;144:763-70. [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [PubMed]
- Masaoka A, Mondren Y, Nokahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Thymectomy and malignancy. Eur J Cardiothorac Surg 1994;8:251-3. [PubMed]
- Muller-Hermelink HK, Strobel P, Zetti A, et al. Combined thymic epithelial tumors. In: Travis WD, Brambilla E, Muller-Hermeling HK, editors. Pathology and genetics: tumors of the lung, pleura, thymus and heart (WHO classification of tumors). Lyon: IARC Press, 2004:196-201.
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998;6 Suppl 2:51S-209S. [PubMed]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii,1-253. [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic diseases. World J Surg Oncol 2014;12:398. [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Rocco G. VATS and Uniportal VATS: a glimpse into the future. J Thorac Dis 2013;5 Suppl 3:S174. [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:7-15. [PubMed]
- Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-9; discussion 1359-60. [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [PubMed]
- Toolabi K, Aminian A, Javid MJ, et al. Mid-term results of thoracoscopic thymectomy for myasthenia gravis. Neurol India 2009;57:402-5. [PubMed]
- Yim AP. Paradigm shift in surgical approaches to thymectomy. ANZ J Surg 2002;72:40-5. [PubMed]
- Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. [PubMed]
- Fleck T, Fleck M, Müller M, et al. Extended videoscopic robotic thymectomy with the da Vinci telemanipulator for the treatment of myasthenia gravis: the Vienna experience. Interact Cardiovasc Thorac Surg 2009;9:784-7. [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [PubMed]
- Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-9; discussion 1359-60. [PubMed]
- Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985;39:165-9. [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Single-centre 40-year results of redo operation for recurrent thymomas. Eur J Cardiothorac Surg 2011;40:894-900. [PubMed]
- Okumura M, Ohta M, Tateyana H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [PubMed]
- Roden AC, Yi ES, Jenkins SM, et al. Diagnostic significance of cell kinetic parameters in World Health Organization type A and B3 thymomas and thymic carcinomas. Hum Pathol 2015;46:17-25. [PubMed]
- Roden AC, Yi ES, Cassivi SD, et al. Clinicopathological features of thymic carcinomas and the impact of histopathological agreement on prognostical studies. Eur J Cardiothorac Surg 2013;43:1131-9. [PubMed]
- Lenihan JP Jr. Navigating credentialing, privileging, and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol 2011;54:382-90. [PubMed]