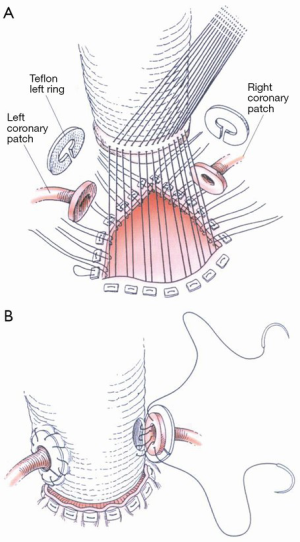
Special considerations in mitral valve repair during aortic root surgery
Introduction
Combined mitral valve repair and aortic root surgery are complex surgical procedures. For mitral valve repair, the required techniques may vary from simple implantation of prosthetic rings, to complex reconstruction in patients with full-blown Barlow’s disease. Aortic root surgery may also vary from biological or mechanical conduit implantation to reconstructive techniques, such as David or Yacoub procedures. Often these patients also need extended aortic surgery, such as replacement of the ascending aorta, hemiarch or arch. Long-standing mitral valve disease may necessitate additional surgical procedures, such as occlusion of the left atrial appendage and pulmonary vein ablation.
Concept of the surgical procedure
First, the ascending aorta is opened transversely and the aortic root inspected. Thereafter, the aortic root is prepared and the aortic valve either preserved for DAVID procedure or excised for conduit implantation. From there on, the mitral valve repair is performed. At this stage, it is important to complete all other procedures within the left atrium (e.g., closure of the left atrial appendage, pulmonary vein ablation). Any later manipulation while the two valves are already in place may lead to severe bleeding complications (e.g., atrio-ventricular disconnections). Finally, the aortic root procedure is performed together with replacements of the ascending aorta or aortic arch.
Anatomical relationships and operative techniques
The close anatomical connections between the aortic root and the mitral valve require special consideration when mitral valve repair and aortic root surgery are performed simultaneously.
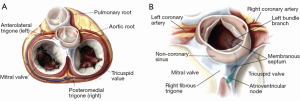
The aortic root is deeply anchored onto the base of the heart between the pulmonary root anteriorly, and the mitral and tricuspid valve posteriorly (1) (Figure 1A,B). The junction between the mitral valve and the aortic root is comprised by the fibrous skeleton of the heart, including the two fibrous trigones (anterolateral and posteromedial).
The mitral valve has four distinct structures, which are close to the annulus (Figure 2):
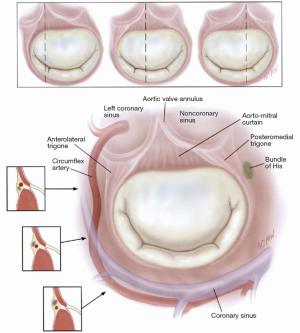
- The circumflex artery, which runs between the base of the left atrial appendage and the P1 segment of the posterior leaflet, usually 3-4 mm from the leaflet attachment;
- The coronary sinus, which skirts the posterior annulus;
- The bundle of His, located near the posteromedial trigone;
- The nadir of the non-coronary and left coronary aortic cusps, located 6-10 mm away from the anterior mitral valve annulus.
Safeguards and pitfalls
Postoperative myocardial failure may be one of the major problems after combined procedures of aortic root and mitral valve. Postoperative myocardial failure is usually due to multiple factors, all of which must be taken into account in the preoperative surgical plan as well as preventative and treatment strategies. Such factors include:
- Patient-related factors: reduced right ventricular ejection fraction (RVEF), reduced left ventricular ejection fraction (LVEF), previous myocardial infarction with regional hypokinesis, akinesis, or dyskinesis and/or pulmonary hypertension;
- Surgery-related factors: long aortic crossclamp time, long cardiopulmonary bypass time, inadequate myocardial protection techniques, coronary artery embolization (air, CO2, atherosclerotic material) and/or inadequate coronary reimplantation in a valved conduit.
To reduce the incidence of postoperative myocardial failure, the following safeguards should be implemented.
Patients with reduced RV function are at high risk for postoperative myocardial failure. The following questions have to be answered preoperatively:
- Is the ventricular septum in midline and contracting well? If not, is the right coronary artery stenosed or occluded? Is the blood supply to the septum restricted in the left anterior descending (LAD), diagonal branches, and/or right coronary artery? If so, plan to revascularize the respective vessels;
- Does pulmonary hypertension exist? If yes, prepare nitric oxide upon weaning the patient from cardiopulmonary bypass;
- Optimize myocardial protection for the right ventricle [antegrade or combined ante-, retrograde blood cardioplegia (BCP), balloon inflated retrograde coronary sinus catheter].
In patients with reduced LV function:
- Is the cause ischemic or dilated cardiomyopathy?
- Evaluate revascularization and/or left ventricular reconstruction if the left ventricular end-diastolic diameter (LVEDD) is >60 mm;
- Use warm blood cardioplegic induction;
- Minimize the cross-clamp time and avoid any second clamp period, i.e., choose mitral valve replacement instead of mitral valve repair;
- Evaluate pre- or intraoperative implantation of the intraaortic balloon;
- When anticipating long pump runs and long aortic cross-clamp times, use ante- and retrograde cold BCP with cold reinfusions every 20 minutes and warm terminal reperfusion shortly before opening the aortic crossclamp. Antegrade delivery of BCP is done for every second reinfusion to allow uniform distribution of the cold cardioplegic solution to all areas of the heart;
- Ensure that the retrograde catheter is in perfect place (self-inflating or balloon-inflated);
- Look for pressures, volume and duration of cardioplegic delivery.
Coronary embolization during aortic root surgery should be avoided by all means. The surgical field should be insufflated with CO2. In cases with heavy calcifications of the cusps, root and ascending aorta, a small sponge should be placed in front of the left coronary ostium to avoid atherosclerotic coronry embolization. In addition, retrograde cardioplegia should be used for “washout” of any embolic material.
Distortion of coronary arteries should be avoided, by using optimal surgical techniques throughout the operation, including reimplantation of the coronary ostia in the aortic prosthesis. Furthermore, beware of injuring the circumflex coronary artery during implantation of the mitral ring (P1 segment) or during endocardial occlusion of the left atrial appendage.
In cases of postoperative myocardial failure, implant an intraaortic balloon (IABP) or an extracorporeal life support system (ECLS).
Inadequate mitral repair after completion of the procedure can be detected in the operating room by transesophageal echocardiography (TEE). Higher grades of mitral valve regurgitation (> grade I-II) after releasing the cross clamp are related to inadequate mitral valve repair. If mitral valve insufficiency remains, this must be corrected. It usually leads to another period of aortic crossclamp in order to redo the repair or replace the mitral valve with the aortic valve already implanted in the aortic root. This in turn increases the danger of bleeding from the aortic root from distortion of the aortic valve and impairs visualization of the mitral valve.
In addition, inadequate mitral valve repair with the necessity of a second aortic cross-clamp period will increase the bleeding tendency after surgery, due to a longer pump run, and may precipitate myocardial failure (especially in patients with reduced LVEF and hemodynamic compromise preoperatively).
Therefore, attempts to repair the mitral valve during an aortic root procedure should only be made in younger patients (<70 years), and if the pathology of the mitral valve allows a safe repair, especially in patients with reduced ejection fractions to start with.
If the mitral valve has to be approached after the aortic root procedure, evaluate which is the best incision for left re-atriotomy, including the standard left atrial approach (Sondergaard’s plane/Waterston’s groove), the transseptal Dubost approach, the transseptal Guiraudon approach, the modified dome approach, and approaching through the roof of the left atrium (3).
A very small aortic root may be a problem, especially if the mitral valve requires repair or replacement. The aortic annulus diameter should have already been measured preoperatively from the CT scan or the TEE. An appropriate strategy plan can be made if the true aortic annulus is known. In particular, beware of smaller and older female patients with a small body surface area. Such patients often have very small aortic roots and mitral annulus’.
In patients with a very small aortic root and annulus, several options exist to either increase the diameter of the anulus or to allow an unobstructed flow from the left ventricle to the aorta:
- Enlargement of the ascending aorta only (incision in the non-coronary sinus until the aortic annulus);
- Enlargement through the aortic annulus, but not in the anterior leaflet of the mitral valve (Nick’s incision);
- Enlargement according to Manouguian and Epting with an incision into the anterior leaflet of the mitral valve associated frequently with opening of the left atrium;
- Replacement of the aortic root;
- Enlargement according to Konno’s method;
- Apico-aortic conduit;
- Supravalvular implantation of the aortic valve with occlusion of the coronary ostia and coronary artery bypass grafting.
An oversized aortic valve prosthesis can be a major problem during this combined procedure with concomitant mitral valve repair. Oversizing the aortic valve prosthesis results from either attempting to implant a 19 mm valve in a very small (<19 mm) aortic annulus, or the largest possible valve to avoid any patient-prosthesis mismatch. For the very small aortic annulus (<19 mm), strategies have to be used to increase the annulus in size for a much larger valve (see below). With regard to the largest possible valve, Denton Cooley once said, “More patients dying from a valve that is too large than from a valve that is too small”. The oversized valve may lead to bleeding from the aortic root or the impossibility to implant that valve, necessitating implantation of a new smaller valve and subsequently, a longer cross clamp period.
In order to avoid oversizing the aortic prosthesis, there are tables available showing the necessary effective orifice area index (EOAI) in relation to the body surface area. In addition, the next bigger valve size has also to pass the aortic annulus after debridement.
Bleeding can be another major problem after surgery and there are many different reasons for this complication. It can result due to any of the following, including the aortic root, coronary button reimplantation, the left atrial appendage (after endo- or epicardial occlusion), suture lines, long bypass time, general bleeding tendency (e.g., warfarin or thrombocyte inhibitors preoperatively). The safeguards shown in Table 1 can be applied to reduce bleeding.
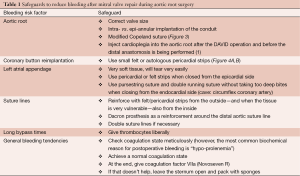
Full table
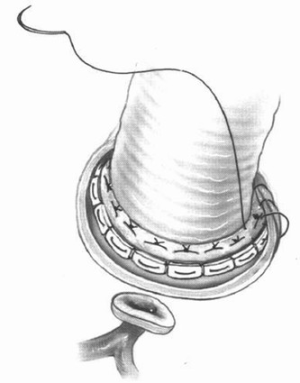
Complete atrio-ventricular block may occur after aortic root surgery. It results from injury of the His bundle near the right and non-coronary annulus during aortic root surgery or from injuring the His bundle during mitral valve surgery near the postero-medial trigone. This problem can only be avoided when taking in account the anatomical relations of the conductive system during aortic root and mitral valve surgery. The surgical technique has to be modified accordingly.
Comments
In all surgical procedures, attention to detail is the most important factor for success. This starts with a well thought-out strategy of each single surgical step in the appropriate sequence. In addition, potential complications have to be kept in mind, as well as the correct bail-out procedure for each problem.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bayfield MS, Kron IL. Reducing bleeding after replacement of the aortic root. Ann Thorac Surg 1995;60:1130-1. [PubMed]
- Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery. Saunders Elsevier, 2010.
- Hussain ST, Alsalihi M, Blackstone EH, et al. Maximized left atrial dome approach for left atrial tumor resection. J Thorac Cardiovasc Surg 2014;148:748-50. [PubMed]
- Khonsari S, Sintek CF. Cardiac Surgery: Safeguards and Pitfalls in Operative Technique. 4th Edition. Wolters Kluwer/Lippincott Williams & Wilkins, 2008.