Designing innovative retractors and devices to facilitate mitral valve repair surgery
Full sternotomy setting
During mitral valve surgery such as mitral valve plasty (MVP) or mitral valve replacement (MVR), optimal exposure of the mitral valve is crucial (1) . In a full sternotomy setting, retractors are designed to horizontally retract the edge of the left atrium to the left side of the patient. This allows the surgeon to visualize the mitral valve vertically from in front of the patient’s body. These retractors are attached to the sternal retractor using various arms and connectors. The connections are designed with low-profile parts that facilitate surgical manipulations, including suturing.
The posteromedial wall of the left atrium tends to obscure the surgeon’s line of sight, especially of the P2-P3 area of the mitral annulus. To facilitate viewing this area, a retracting arm is usually optional, and may be fixed to a crossbar of the sternal retractor with a special connector.
In the full sternotomy setting, the left atrial retractor is designed for optimal exposure of the mitral valve, suture management, and support by an assistant on the opposite side of the patient. One of the purposes of the left atrial retractor is to allow the assistant to free one or both hands to concentrate on suture management and other tasks for the surgeon. The Cor-Valv retractor (Coroneo, Montreal, QC, Canada) (2), which is fixed to a sternal retractor, features three adjustable fingers to provide varying widths of tissue retraction. A surgeon can directly control the position and orientation of this retractor to optimize mitral valve exposure. This system allows the assistant to concentrate on other aspects of the surgery without any concern about mitral valve exposure.
Mini-mitral surgery
Although minimally invasive mitral valve surgery (MIMVS) is an accepted treatment option for patients with mitral valve disease, the majority of surgeons remain unfamiliar with it because specialized training is required and practice is needed to maintain proficiency (3). Innovative surgical instruments have been developed to mitigate the challenges of MIMVS. For MIMVS via minithoracotomy, an innovative retractor design is particularly important, as the help from an assistant to enhance valve exposure is limited due to the smaller working space, and the fact that the assistant is on the opposite side of the patient. Thus, the surgeon alone is responsible for maintaining optimal exposure of the mitral valve. Various left atrial retraction systems have been developed by expert MIMVS surgeons, and are broadly classified into two categories. One category encompasses those of the trans-thoracic wall type (4), in which a shaft, fixed to a blade, is inserted through an intercostal space into the thoracic cavity. The second category refers to the trans-working-port type, in which the shaft is placed through the working port without a stab wound.
Trans-thoracic wall retractor
Generally, the trans-thoracic wall retractor provides very stable mitral valve exposure because the trans-thoracic shaft is stabilized with a support system fixed to the surgical bed. The HV Heart retractor (USB Medical, Huntingdon Valley, PA, USA) (5) is the signature trans-thoracic wall type retractor for MIMVS. It features a self-adjusting pivot blade with an adjustable angle mechanism and a self-wiping hinge. The device is inserted under videoscopic guidance through the fourth intercostal space, just lateral to the right internal mammary vein. The self-adjusting pivot blade also eliminates stress on the heart during retraction. The blade itself is hinged and has an adjustable angle system that provides great exposure regardless of anatomic variance. The self-wiping hinge also retracts the left atrial wall at the posteromedial commissural area of the mitral valve. In light of the abundant experience with endoscopic MIMVSs (6), these valuable mechanisms have been integrated and work very well in the limited working space available in these procedures. Although this instrument provides surgeons with stable exposure of the mitral valve, this system also carries the potential risk of injury to the mammary or intercostal vessels when the retractor shaft penetrates the chest wall. Additionally, the positioning of the shaft’s point of penetration through the chest wall affects mitral valve exposure. To achieve satisfactory exposure, sufficient knowledge and experience with the instrument is necessary.
Because the retractor is not placed through the working port, determination of the site of the working port is independent of considerations for proper retraction. Furthermore, the working port remains free of obstruction, thereby reducing the risk of suture jamming around the working port. During endoscopic MIMVS, making a simple and orderly working port is very important for obtaining optimal surgical results because the surgeon can concentrate on the endoscopic view, without consideration for what is transpiring outside of the working port.
Trans-working-port retractor
In contrast to the trans-thoracic wall retractor, the trans-working-port retractor is a versatile tool because the blade can be placed anywhere the surgeon desires due to the flexible movement of the shaft. Although the blade is restricted by the edge of the working port, it is not restricted as a result of its insertion through a small hole in the chest wall. Avoiding blood loss from the mammary or intercostal vessels is another important advantage of this type of retractor. To properly expose the mitral valve, a retractor should be flexible and stable, and its handling should be familiar. The Adams-Yozu Mini-Valve System (Geister, Tuttingen, Germany) (7) is an optimal retraction tool for MIMVS (Figure 1). Utilized in over 200 MIMVSs over a 12-year period at Keio University (Tokyo, Japan), the retractor is specialized for MIMVS and has solved multiple mitral valve exposure difficulties.
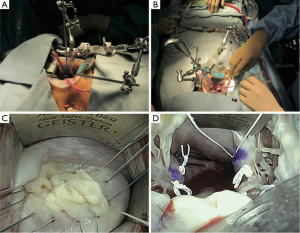
This retractor includes a minithoracotomy tissue spreader, a left atrial blade, and an additional bendable retractor for the posteromedial wall (P3 area) of the left atrium. The main minithoracotomy spreader is designed to fit the ribs, and the bar between the blades is angled. When the spreader is gradually opened, the cranial blade elevates the upper rib. To insert and remove the aortic root cannula under direct vision in MIMVS, a surgeon must access the ascending aorta through the small working port in the fourth or fifth intercostal space. The retractor’s elevating system widens exposure of the ascending aorta to facilitate aortic manipulation.
The primary feature of this mini-valve system is its flexibility. The left atrial blade is connected to the minithoracotomy spreader via a detachable, multi-joint arm. It can be moved to various positions and allows for optimal positioning of the retractors, depending on the surgeon’s preference. Although the small or medium retractor is suitable for average left atrial sizes, five blade sizes are available for use to accommodate for variance in the size and the anatomy of the target region. Because detachment of the shaft from the multi-joint connector is easy and the shaft has sufficient length to place a handgrip at the level of the surgeon’s arm, the left atrial retractor can be easily exchanged for another size with one hand. The papillary muscles are easily visualized and reached with a straight view through the small working port. This view is achieved by stably retracting the anterior leaflet of the mitral valve toward the anterior side using the smallest blade, which is deeply inserted into the left ventricle. With the anterior leaflet retractor in place, an additional side arm may further enhance exposure of the papillary muscles by posteriorly retracting the posterior leaflet. As such, secure and comfortable manipulation of the papillary muscles for the creation of neo-chordae with expanded polytetrafluoroethylene (ePTFE) suture is facilitated.
Usually, exposure of the P2–P3 area is difficult because of the challenge associated with elevating the posterior wall of the left atrium. To solve this problem, an additional retractor, attached to the minithoracotomy spreader, can push the left atrial wall out of the surgeon’s field of view. In the case of inadequate exposure of the P1 area, the additional retractor can also be used to retract the anterolateral side of the left atrial wall. Because the Adams-Yozu retractor is a universal device, it is familiar to surgeons who perform MIMVS directly or endoscopically. Even in full sternotomy cases, this system can attach to the sternotomy retractor with a special connector. Although tricuspid valve annuloplasty (TAP) is generally difficult to perform in a minithoracotomy setting, exposure of the tricuspid valve is easy when taking advantage of the flexibility of the Adams-Yozu retractor. The device’s disadvantages are its high profile and complicated construction, which can contribute to suture jamming and collisions with long-shafted instruments. However, with a little ingenuity, these challenges can be overcome. Another disadvantage of this system is that because the retractor is fixed to the minithoracotomy spreader, the retractor is moved whenever the spreader is moved during surgical maneuvers, making this type of retractor less stable than retractors fixed to the surgical bed.
Since the launch of Adams-Yozu Mini-Valve System in 2010, this device has been used in 205 MIMVSs (186 MVPs and 19 MVRs, including 16 additional TAPs) at Keio University. A right minithoracotomy in the fourth or fifth intercostal space with a 6 to 8 cm skin incision is typically used as a working port, and cardiopulmonary bypass is established with peripheral cannulation of the femoral artery and vein, as well as the jugular vein (8). The ascending aorta is clamped with a Cosgrove flexible clamp through the working port or with a transthoracic Chitwood clamp. Cold cardioplegia is infused via a three-lumen cardioplegia needle to achieve cardiac arrest and the left atrium is then opened through Waterston’s groove. No procedure required conversion to sternotomy since the use of the device was implemented. To date, the mean total surgical and aortic cross-clamping times (AOT) are 353.5±79.1 (range, 181-635) minutes and 180.2±50.0 (range, 68-348) minutes, respectively. Comparing isolated MVPs without TAP between cases performed during the first three years and after, the AOTs have significantly decreased: 191.1±44.1 (range, 102-323) minutes in the earlier cases and 159.4±47.0 (range, 84-277) minutes in the later cases. This data shows that familiarity with the retractor and knowledge of how to expose the mitral valve permits facile manipulation of the mitral valve, translating into shortened surgical times.
Alternatives
To enhance the use of MIMVS and to simplify the surgical field, a simple, low-cost, low-profile retractor can be utilized. A single, orthogonal steel plate with holes and rounded, non-traumatizing edges, suspended with two strong prolene sutures (Ethicon, Somerville, NJ, USA) can function as a left atrial retractor (9). The prolene sutures penetrate the chest wall to optimally expose the mitral valve. The exposure can be adjusted by changing the position of the sutures thorough the chest wall. This very simple device is an easy to use, flexible tool. A similar concept for a low-cost retractor is the use of a prolene suture with a pledget positioned on the posteromedial wall of the left atrium (10). This device serves as a strong tool for exposing the posteromedial commissure of the mitral valve by plicating of the left atrium. A combination of these tricks and commercial devices may facilitate MIMVS for even the most complex cases of valve exposure.
A pattern-cut polymer sheet, deployed and secured with a pivoting rivet, generates a conical volume, and is called a MitraXs retractor (St Jude Medical, Minneapolis, MN, USA). This device is a novel left atrial retractor specifically dedicated to MIMVS (11). The device is a self-expanding, auto-adjusting, and single-use left atrial retractor that does not require a supporting arm. The device maintains an optimal conical shape that when expanded keeps the left atrium wide open, in a symmetrical manner. Although the MitraXs device generally provides optimal exposure and excellent direct vision, it has not yet gained popularity and widespread utilization.
Devices to support neo-chordae creation
Creating neo-chordae from ePTFE sutures is very challenging for surgeons because tying the slippery ePTFE sutures at the proper point is very difficult. Furthermore, protecting an ePTFE suture from damage during fixation of the knot is important because any damage to the suture may cause suture breakage and affect the long-term results of neochordal based repairs. To assist in making knots that do not slip, various devices have been developed. To compare the lengths of the neo-chordae with native chordae, a specially designed, double-headed retractor was developed for mitral leaflet retraction (Senko Medical Instrument Manufacturing, Tokyo, Japan) in which both mitral leaflets are simultaneously retracted to the same level (12). Additionally, the device can be attached to a Hercules Universal Stabilizer Arm (Estech, Danville, CA, USA) to support appropriate suture tying. A Stop-Slip Pipe for neochordal reconstruction (Geister) works in a different fashion. When a surgeon ties an ePTFE suture on the mitral leaflet, the stop-slip pipe, which is placed between the head of the papillary muscle and the top of the mitral leaflet, effectively prevents knot slippage. The length of the pipe can be chosen from three sizes (18, 21 or 24 mm) according to preoperative echocardiography or CT images.
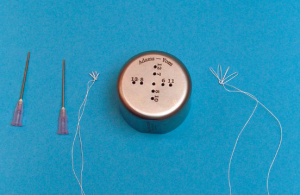
Carpentier’s gold standard resection-based techniques are difficult when utilizing a minimally invasive approach. Thus, surgeons tend to prefer neochordal based repairs to manage the prolapsed posterior leaflet. Multiple manipulations of the papillary muscle are necessary in cases with broad prolapsed lesions or bi-leaflet disease. However, multiple manipulations of the papillary muscle, during MIMVS, are technically difficult and even dangerous due to the small working space and the distal manipulations that require the use of long-shafted devices. To repair mitral valves with multi-segment prolapse via a right minithoracotomy, the loop technique (13,14) is facilitated by a tool designed especially for use during MIMVS. A loop-maker is useful in facilitating the creation of an appropriately sized ePTFE loop. In the Shibata Chordae System (Geister), 15 to 26 mm ePTFE loops can be easily made (15). An ePTFE suture is passed through a pledget and is turned at the rod standing at the location of the desired length, creating a loop. The suture is again passed through the pledget, and tied 6 to 8 times to complete the first loop. The desired numbers of loops are made in a similar manner. The pledget, with ePTFE loops, is removed from the pledget holder by pushing a knob. A round loop maker, with several holes (Geister) is a new device for creating loops (16). A surgeon places 18 gauge needles into two holes, according to the desired length of the ePTFE loops, and the loops are made by tying the ePTFE sutures around the needles (Figure 2). Once fashioned, the loop can be released without any damage to the ePTFE suture. Although a very simple device, it enables a surgeon to make loops of various lengths using a combination of holes.
Conclusions
Various devices have been developed to facilitate mitral valve surgery, including those that enhance mitral valve exposure as well as those that assist with difficult aspects of repair procedures. Choosing appropriate supporting devices and surgical strategies is paramount, particularly when performing thoracoscopic MIMVS via a right minithoracotomy. A sound understanding of retractors and surgical techniques is key in developing a reproducible and safe surgical system for mitral valve surgery.
Acknowledgements
Dr. David Adams (Mount Sinai School of Medicine, New York, NY, USA) supervised the development of the Adams-Yozu Mini-Valve System and provided us with many ideas and suggestions.
Disclosure: The authors declare no conflict of interest.
References
- McCarthy JF, Cosgrove DM 3rd. Optimizing mitral valve exposure with conventional left atriotomy. Ann Thorac Surg 1998;65:1161-2. [PubMed]
- COR-VALV system. Available online: http://www.coroneo.com/coroweb/index.php/products/5-cor-valv-system
- Misfeld M, Borger M, Byrne JG, et al. Cross-sectional survey on minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:733-8. [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. From classical sternotomy to truly endoscopic mitral valve surgery: a step by step procedure. Heart Lung Circ 2003;12:172-7. [PubMed]
- USB MEDICAL. Available online: http://www.usb-medical.com
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [PubMed]
- Yozu R, Okamoto K, Kudo M, et al. New innovative instruments facilitate both direct-vision and endoscopic-assisted mini-mitral valve surgery. J Thorac Cardiovasc Surg 2012;143:S82-5. [PubMed]
- Kudo M, Yozu R, Kokaji K, et al. Examination of mitral valve repair with port-access method--Aiming at early and less invasive mitral valve repair--. Gen Thorac Cardiovasc Surg 2009;57:298-302. [PubMed]
- Kofidis T, Lee CN. A novel and simple atrial retractor. Ann Thorac Surg 2011;91:1634-5. [PubMed]
- Ng CK, Benedikt P, Schwarz C, et al. Self-retaining pledgeted suture as retractor for mitral procedure. Asian Cardiovasc Thorac Ann 2009;17:206-7. [PubMed]
- Jegaden O, Sassard T, Shafy A, et al. Evaluation of a new left atrial retractor for minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg 2011;141:297-9. [PubMed]
- Kashiyama N, Masai T, Yoshitatsu M, et al. A simple way to treat mitral valve prolapse: chordal replacement using a new mitral leaflet retractor. Interact Cardiovasc Thorac Surg 2014;18:701-5. [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [PubMed]
- Seeburger J, Kuntze T, Mohr FW. Gore-tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg 2007;19:111-5. [PubMed]
- Shibata T, Inoue K, Ikuta T, et al. A workbench to make artificial chordal loops for mitral valve repair. J Thorac Cardiovasc Surg 2009;138:506-7. [PubMed]
- Okamoto K, Yozu R, Kudo M. Loop-in-loop technique in mitral valve repair via minithoracotomy. Ann Thorac Surg 2012;93:1329-30. [PubMed]





