Thoracoabdominal aortic aneurysm: hybrid repair outcomes
Background: Thoracoabdominal aortic aneurysms (TAAA) remain amongst the most formidable of surgical challenges, particularly degenerative aneurysms in the elderly population with concomitant pulmonary disease. This report presents an update of our robust single-institution experience with “hybrid” TAAA repair including complete visceral debranching and endovascular aneurysm exclusion in high-risk patients.
Methods: Between March 2005 and June 2012, 58 patients underwent extra-anatomic debranching of all visceral vessels followed by aneurysm exclusion via endovascular means at a single institution. The median number of visceral vessels bypassed was 4. The debranching and endovascular portions of the procedure were performed as a single stage in the initial 33 patients and as a staged approach in the most recent n=25 cases.
Results: Median patient age was 69.0 years; 50% were female. All had significant co-morbidity and were considered suboptimal candidates for conventional open surgical repair. Mean aortic diameter was 6.7±1.2 cm. Thirty-day/in-hospital rates of death, stroke, and permanent paraparesis/paraplegia were 9%, 0%, and 4%, respectively; in the most recent 25 patients undergoing staged repair these rates were 4%, 0%, and 0%. Over a mean follow-up of 26±21 months, visceral graft patency is 95.3%; all occluded limbs were to renal vessels and none resulted in permanent dialysis. Two patients (3%) have required re-intervention, one for type Ib and one for type III endoleak. Five-year freedom from re-intervention was 94%. Kaplan-Meier overall survival was 78% at 1 year and 62% at 5 years, with a 5-year aorta-specific survival of 87%.
Conclusions: These updated results continue to support hybrid TAAA repair via complete visceral debranching and endovascular aneurysm exclusion as a good option for elderly high-risk patients less suited to conventional open repair. A staged approach to debranching and endovascular aneurysm exclusion appears to yield optimal results.
Key words: Thoracoabdominal aortic aneurysms (TAAA); pulmonary disease; endovascular aneurysm
Introduction
Thoracoabdominal aortic aneurysms (TAAAs) remain a formidable surgical challenge with conventional open repair associated with significant rates of mortality and morbidity in the average center (1). Two-thirds of these aneurysms are degenerative (atherosclerotic) in nature and typically occur in the elderly with significant co-morbidities, most commonly chronic obstructive pulmonary disease. This population is particularly poorly suited for conventional repair (2), and the search for an alternative approach to managing these patients has led to the development of the so-called “hybrid” method of repair involving extra-anatomic bypass of the visceral vessels (“debranching”), with subsequent endovascular exclusion of the aneurysmal pathology (3,4). This hybrid TAAA repair procedure uses currently available off-the-shelf thoracic and abdominal endovascular devices and familiar surgical techniques, and has become the procedure of choice in our center for patients deemed high risk for conventional open TAAA repair. The current paper represents an update of our single-center experience (5) with total visceral debranching and endovascular repair for TAAAs.
Methods
Patients and data source
A prospective cohort review was performed of all patients (n=58) undergoing hybrid repair involving complete visceral debranching and endovascular aneurysm exclusion for Crawford extents I, II and III TAAAs between March 2005 (date of FDA approval of the first available thoracic device in the U.S.) and June 2012 at a single referral institution. Preoperative, intraoperative, and postoperative variables were abstracted from the Duke Thoracic Aortic Surgery Database, which is a prospectively maintained clinical registry of all patients undergoing thoracic aortic surgery at Duke University Medical Center (Durham, NC) since 2005. The study was reviewed and approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived. General criteria regarding patient selection for “hybrid” over conventional TAAA repair have been described previously (3,5) and include age >65 years, cardiac disease, pulmonary disease, renal insufficiency, and prior open abdominal or descending/TAAA repair. These criteria are relative factors in the decision-making process and not absolute indications/contraindications. Ideally, the decision for conventional versus “hybrid” repair should be made by a surgical team with expertise in both techniques and consideration of institutional results with each technique should factor heavily into the decision making process (3). We consider the presence of a connective tissue disorder to be a contraindication to this approach unless proximal and distal landing zones are completely within existing Dacron grafts (6). Crawford extent IV aneurysms are treated with conventional open repair at our institution and are not included in this report.
This report includes all data collected through the patients’ most recent follow-up visit. In addition, the social security death index was queried (http://ssdi.rootsweb. com/) to confirm all patient deaths, including patients not returning for follow-up visits. For those patients dying in follow-up, cause of death was confirmed by review of medical records or family interview in all cases. Survival analyses were performed using the Kaplan-Meier method. All data are presented in accordance with the “Reporting standards for thoracic endovascular aortic repair (TEVAR)” of the Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards (7).
Operative technique
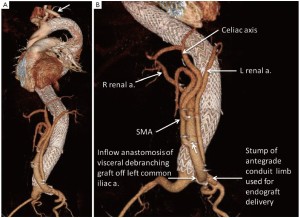
Although originally performed in a single stage to include both the debranching and endovascular portions of the procedure (3,4,8), the repairs are now done in a staged fashion with the open abdominal portion performed first and the endovascular portion 3-7 days later during the same hospitalization. Details regarding the technique of visceral debranching have been previously described in detail (5). Briefly, a midline laparotomy incision is performed, and the right renal artery approached first by incising the peritoneum lateral to the third portion of the duodenum and exposing the inferior vena cava (IVC). The right renal vein is identified where it enters the IVC, and the artery is usually found cephalad and deep to it. The preoperative imaging is studied carefully to insure the main trunk of the artery is controlled if there is early branching. Attention is then turned to the infrarenal aorta, which is exposed via a standard dissection of the anterior surface up to the level of the left renal vein. The proximal superior mesenteric artery (SMA) and celiac axis are next exposed above the left renal vein by elevating the pancreas. These vessels are dissected over adequate distance to permit clamping, division, and secure closure of the aortic stump. The aorta at the origins of these vessels is aneurysmal and often friable, so extreme care must be taken to insure an adequate and secure closure. This often includes oversewing with pledgeted vascular sutures. Finally the left renal artery is dissected free, which may require division of some branches of the left renal vein. The proximal inflow anastomosis for the multibranched visceral bypass graft utilized for debranching can be to the distal aorta, either iliac system if large enough, or a previously placed aortic graft or iliac limb; the left common iliac artery is the most common inflow source.
A commercially available graft (Vascutek, Ann Arbor MI, USA) designed specifically for this operation is used (Figure 1). The graft has a 14-mm trunk, two 6-mm side-limbs for the renal arteries, and two 8-mm side limbs for the visceral (celiac, SMA) branches. In addition there is a 10-mm side-limb at the proximal end adjacent to the inflow anastomosis that is used as a conduit for the large sheaths during the delayed second endovascular stage. This generally avoids the need for additional vascular exposure at this second operation. At the end of the first operation, just before closing, the conduit limb designed for endograft introduction is exited in a retroperitoneal tunnel to a pocket on the abdominal wall that is positioned to afford as straight-line access to the aorta as possible. Each branch of the debranching graft has radiographic markers for identification should angiography of the bypass graft be required in the future.
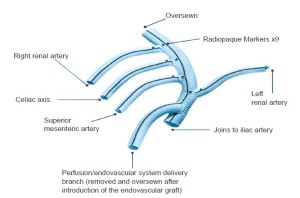
Following completion of the vascular exposures, the patient is heparinized (100 u/kg) to a target ACT >200 seconds. The proximal inflow anastomosis is done first, after trimming the graft obliquely so as to minimize the angle of entry of the endovascular access limb to the aorta. The origin of the bypass graft is marked with radiographic markers to allow identification of the graft origin under fluoroscopy and aid in deployment of the endovascular grafts as the radiographic markers indicate the most distal position beyond which the endografts should not extend so as to avoid compromising the inflow to the visceral debranching graft. The branches of the graft are then sequentially anastomosed to their respective visceral branches in end to end fashion. In this manner, visceral ischemia time is minimized and usually less than 10 minutes per anastomosis. After completing the three left sided bypasses, the right renal graft is passed through the root of the mesentery over the aorta and IVC to the right retroperitoneal exposure. After completion of all anastomoses, the heparin is reversed with protamine and other clotting factors are given as required. The graft can usually be covered with peritoneum to avoid adhesion of the bowel to the graft. After storing the access limb in a small subcutaneous abdominal wall pocket, the abdomen is closed in standard fashion.
Post-first stage debranching, the patient is monitored overnight in the cardiothoracic intensive care unit. They are maintained well hydrated, and bowel function, renal function, and blood pressure are monitored. The second stage is performed 3-7 days later to permit recovery of renal function and avoid simultaneous renal stress with contrast injection. Patients remain in the hospital between stages to reduce the risk of interval rupture through a monitored care environment and by minimizing the time interval between procedures. Both the first and second stages are done in a hybrid operating room with fixed imaging equipment and full operative capability.
Details regarding the technique of second stage endovascular repair have been previously described in detail (5). To start the endovascular second stage, the access limb of the debranching graft is exposed by opening the abdominal wall pocket. The graft limb is pulled out of the pocket and thrombectomized. The only other vascular access that is required for this stage (unless a bifurcated distal device is utilized, in which case unilateral groin exposure will be required as well) is a percutaneously placed 5-French sheath in either femoral artery for passage of the diagnostic angiographic catheter. The aneurysm is then relined by deploying any of the available thoracic (+/- abdominal) endograft systems. The large bore access sheath is inserted only a small distance into the access limb of the visceral debranching graft to avoid occlusion of the main channel feeding the viscera during endograft delivery. After endograft deployment and completion arteriogram of the aorta, the debranching limb is selectively imaged to confirm visceral and renal graft limb patency. After completion of the endovascular procedure, the access limb is amputated and buried beneath the abdominal wall.
For patients with Crawford extent I or II TAAAs requiring left subclavian artery coverage as part of the repair, the left subclavian artery is revascularized for a spinal cord protection indication (9) via a left common carotid to left subclavian bypass at the beginning of the second stage procedure. The endovascular procedure is done using somatosensory and motor evoked potential electrophysiological monitoring of spinal cord function as previously described (10), routine lumbar cerebrospinal fluid drainage, and invasive cardiovascular monitoring in the event that evoked potential evidence of spinal cord ischemia requires manipulating the blood pressure or draining of spinal fluid.
Data analysis
Univariate comparisons of preoperative, operative, and postoperative variables were performed between patients undergoing simultaneous or staged aneurysm repair. Continuous variables were compared using non-parametric Wilcoxon’s sign test, and categorical variables were assessed by the chi-squared test or Fisher’s exact test. A probability value of less than 0.05 was considered statistically significant. Unadjusted survival estimates were calculated to produce a Kaplan-Meier curve for overall and aorta-specific survival, as well as simultaneous vs. staged survival, using the log-rank test. All survival analyses were conducted using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Patient demographics
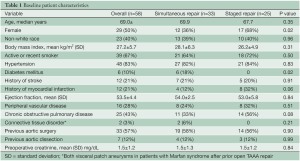
Baseline patient characteristics are presented in Table 1 and operative and procedural characteristics in Table 2. Data are presented for the overall group as well as for simultaneous versus staged repair. Simultaneous repair was utilized in the initial 33 patients while a staged approach performed in the most recent 25 cases; this was performed during a single hospital stay in 24 of the 25 patients. The second stage procedure was performed during a delayed second hospitalization 9 weeks later in a single patient with chronic renal insufficiency who developed transient acute renal failure following first stage visceral debranching; the second stage repair was performed following renal recovery. There were no differences in baseline characteristics between patients undergoing simultaneous versus staged repair. In the staged group no patient failed to complete the second endovascular stage.
Full table

Full table
Procedural (30-day) outcomes
All patients in this series had debranching of all patent visceral vessels; a total of 214 visceral bypasses were performed in the 58 patients for a mean of 3.7 bypasses per patient with the median number of vessels bypassed/patient being 4. The median number of main body endograft components deployed per case was 3 (range, 1-6); 9 (16%) patients required use of a bifurcated abdominal endografting system distally to obtain seal within the iliac arteries due to lack of adequate distal landing zone within the infrarenal aorta.
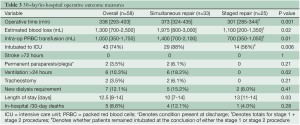
The 30-day/in-hospital operative outcomes are presented in Table 3. Data are presented for the overall group as well as for simultaneous versus staged repair. Operative mortality, defined as death within 30 days of the procedure or during the same hospital admission, was 9% (5/58) for the entire cohort. Rates of stroke and permanent paraparesis/paraplegia were 0% and 4% (2/58), respectively. Results were improved in the most recent 25 patients treated with a staged approach with 4% mortality, and no strokes or paraparesis/paraplegia. Further, operative time, blood loss, transfusion, and mechanical ventilation >24 hours were all significantly reduced with a staged approach (Table 3). Hospital length of stay was longer with a staged approach. No aneurysm ruptures occurred in the interval between the first and second stage procedures in the staged group.
Full table
Follow-up outcomes
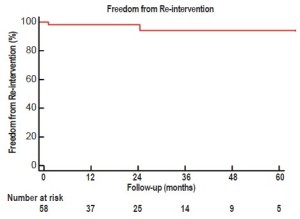
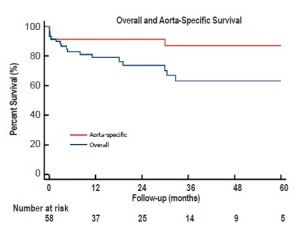
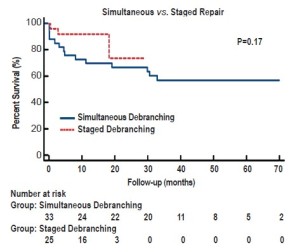
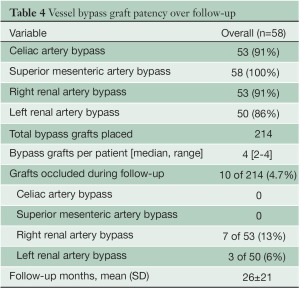
Over a mean follow-up of 26+21 months (range, 2-79 months), visceral graft patency is 95.3% (204/214) (Figure 2); all occluded limbs were to renal vessels and none resulted in permanent dialysis (Table 4). Patency was slightly worse for right (87%) versus left (94%) renal bypasses. All graft occlusions were detected on the 1-month follow-up scan with no new graft occlusions developing thereafter. Two patients (3%) have required re-intervention, one for type Ib and one for type III endoleak, at a mean of 21+18 months postoperatively. Five-year freedom from re-intervention was 94% (Figure 3). Kaplan-Meier overall survival was 78% at 1 year and 62% at 5 years, with a 5-year aorta-specific survival of 87% (Figure 4). There is no difference in late survival between those treated with a staged versus simultaneous approach (Figure 5), although none of the staged patients has follow-up out to three years.
Full table
Discussion
There are three competing strategies for surgical management of extent II and III TAAAs. Open repair, usually with cardiopulmonary bypass and direct reconstruction of the visceral segment is a durable operation (2), but one that entails a significant surgical stress and is poorly tolerated by older patients and those with significant co-morbidities. Poor results in this group have led to a search for less stressful procedures. A totally endovascular approach with endovascular side branches for each of the visceral branches has been described. Although commercial devices for pararenal aortic aneurysms are on the threshold of availability, devices for extent II and III aneurysms with large aneurysmal components of the visceral segment are much further away from being available in the United States. Furthermore, endovascular bypass of all four of the visceral vessels requires a degree of endovascular expertise and sophisticated imaging capability that few centers will be able to possess. The third strategy, a hybrid of open repair but with limited surgical stress, no cardiopulmonary bypass or hypothermia, and no aortic cross clamp in conjunction with endovascular repair with currently available devices has been attractive to our group. It uses familiar surgical techniques, currently available endovascular devices with proven efficacy, and can be applied to patients with significant physiologic limitations. The current report updates our extensive single-center series of this “hybrid” TAAA repair via open visceral debranching with endovascular aneurysm exclusion (5). The results continue to demonstrate the hybrid approach utilized to be a safe alternative to conventional repair in older patients with significant co-morbidity. Further, these updated data further confirm our prior assertion that a staged approach to hybrid repair with the visceral debranching and endovascular portions of the procedure performed 3-7 days apart during a single hospital day yields superior 30-day results. Whether these superior short-term outcomes will be maintained over longer duration follow-up must await further maturation of the data (Figure 5).
The results presented herein have not been uniformly replicated by other centers performing hybrid TAAA repair (11) and reasons for the disparate results reported in the literature are unclear. Clearly, patient selection is important as previous work from our institution has demonstrated that age >75 years, aortic diameter >6.5 cm, American Society of Anesthesiologists class 4, baseline creatinine >1.5 mg/dL, and congestive heart failure are all independently associated with 1-year mortality after thoracic endovascular aortic repair, and that these characteristics identify patients unlikely to derive a longterm survival benefit from the procedure (12). As yet unpublished results from the North American Complex Abdominal Aortic Debranching Registry (13) demonstrated a wide variation in mortality rates among large volume centers and confirmed the importance of patient selection with advanced age, coronary artery disease, congestive heart failure, and renal insufficiency, among others, being associated with increased mortality. Similar to the results of the current study, single-stage procedures were associated with increased mortality in that registry.
In summary, hybrid TAAA repair with visceral debranching followed by endovascular aneurysm exclusion remains a good option for elderly, high-risk patients less suited for conventional open repair in centers with the requisite expertise to care for these complex patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lin PH, Kougias P, Bechara CF, et al. Clinical outcome of staged versus combined treatment approach of hybrid repair of thoracoabdominal aortic aneurysm with visceral vessel debranching and aortic endograft exclusion. Perspect Vasc Surg Endovasc Ther 2012;24:5-13.
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81.
- Hughes GC, McCann RL. Hybrid thoracoabdominal aortic aneurysm repair: concomitant visceral revascularization and endovascular aneurysm exclusion. Semin Thorac Cardiovasc Surg 2009;21:355-62.
- Hughes GC, Nienaber JJ, Bush EL, et al. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2008;136:21-8, 28.e1-6.
- Hughes GC, Barfield ME, Shah AA, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg 2012. [Epub ahead of print].
- Hughes GC. Aggressive aortic replacement for Loeys- Dietz syndrome. Tex Heart Inst J 2011;38:663-6.
- Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 2010;52:1022-33, 1033.e15.
- Hughes GC, McCann RL, eds. Visceral debranching techniques for hybrid thoracoabdominal aortic aneurysm repair. In: Davies AH. Vascular and endovascular surgery highlights 2009-10. Oxford: Health Press Limited, 2010:84-93.
- Lee TC, Andersen ND, Williams JB, et al. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg 2011;92:97-102; discussion 102-3.
- Husain AM, Swaminathan M, McCann RL, et al. Neurophysiologic intraoperative monitoring during endovascular stent graft repair of the descending thoracic aorta. J Clin Neurophysiol 2007;24:328-35.
- Oderich GS, Mendes BC, Gloviczki P, et al. Current role and future directions of hybrid repair of thoracoabdominal aortic aneurysms. Perspect Vasc Surg Endovasc Ther 2012;24:14-22.
- Shah AA, Craig DM, Andersen ND, et al. Risk factors for 1-year mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Oderich GS, Glovicki P, Farber M, et al. Abdominal debranching with aortic stent grafts for complex aortic aneurysms: preliminary results of the North American complex abdominal aortic debranching (NACAAD) registry. Presented in part at the 2011 Vascular Annual Meeting.





