Open reconstruction of thoracoabdominal aortic aneurysms
Introduction
The technical details of our strategy for operating on thoracoabdominal aortic aneurysms are presented below with our emphasis on the importance of reconstructing intercostal and visceral arteries to prevent postoperative paraplegia, intestinal ischemia, and renal failure.
Patients and methods
Between October 1999 and June 2012, 152 patients underwent surgery for thoracoabdominal aortic aneurysms at the Kobe University Hospital [Crawford classification type I=21 (13.8%), type II=43 (28.3%), type III=73 (48.0%), type IV=15 (9.9%)]. Mean age was 64.6±13.9 years and 115 (75.7%) patients were male. Sixty-three (41.4%) had aortic dissection, including 2 (1.3%) patients with acute type B dissection, and 17 (11.2%) had ruptured aneurysms. Eight (5.3%) patients had mycotic aneurysm, and 3 (2.0%) had aortitis. Emergent or urgent surgery was performed in 25 (16.4%) patients. Preoperative computed tomography (CT) scan or magnetic resonance (MR) angiography detected the Adamkiewicz artery in 103 (67.8%) patients. Cerebrospinal fluid drainage (CSFD) was performed in 115 (75.7%) patients and intraoperative motor evoked potentials (MEPs) were recorded in 97 (63.8%) patients. One hundred and seven (70.4%) patients had reconstruction of the intercostal arteries from T7 to L2, using the aortic patch technique in 35 patients and branched grafts in 72 patients. The mean number of reconstructed intercostal arteries was 3.1¡À2.5 pairs. Mild hypothermic partial cardiopulmonary bypass with tympanic temperatures between 32-34 °C was used in 105 (69.1%) patients, left heart bypass was used in 4 (2.6%), and deep hypothermic cardiopulmonary bypass with tympanic temperature <20 °C was used in 42 (27.6%).
Surgical procedure
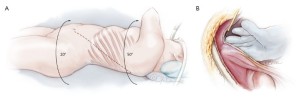
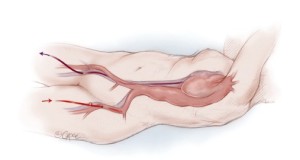
The day prior to the operation, a CSFD catheter is inserted in the lumbar region. On the day of surgery, both groins are incised and right common femoral vein and left common femoral artery are exposed in the supine position. Then the patient is turned into the left semi-posterolateral position with the shoulders rotated rightward 50 degrees and hips rotated 20 degrees (Figure 1A). A catheter for paravertebral nerve block is inserted before skin incision. The skin incision follows a rather straight line. The whole thoracoabdominal aorta is exposed through the left 5th or 6th intercostal space, and a retroperitoneal approach is undertaken. The diaphragm is excised circumferentially, leaving a 2 cm peripheral margin (Figure 1B). Transcranial stimulated MEPs are recorded throughout the operation. Partial cardiopulmonary bypass is initiated through a left femoral arterial cannula and a right femoral venous cannula, which is passed to the inferior caval-atrial junction (Figure 2). Segmental-staged aortic clamping is liberally used under partial cardiopulmonary bypass. Aortic segments containing fewer than three pairs of intercostal arteries are sequentially clamped and opened. The aorta is initially clamped proximal to the left subclavian artery and across the mid-descending thoracic aorta (Figure 3).
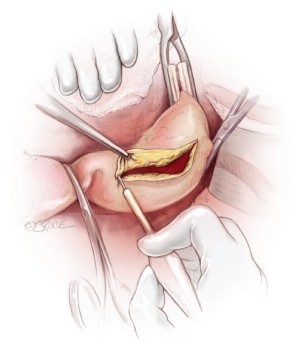
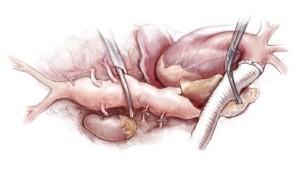
Next, the aorta is incised between the clamps and bleeding orifices from the bronchial arteries or high intercostal arteries are oversewn (Figure 4A). The proximal aorta is completely transected and dissected away from the surrounding esophagus, left vagus nerve, and away thoracic duct (Figure 4B). A 20 or 22 mm Dacron graft with 8-mm side-branches is used. This graft has 4 spatially orientated branches for the abdominal visceral arteries. The proximal anastomosis is performed using a 4-0 monofilament suture (22-mm needle) with Teflon strip reinforcement (Figure 5A). The edge of the Teflon strip is tightened with a compacting stitch to prevent suture hole bleeding (Figure 5B). The descending aorta is then clamped at the T10 level. Before opening the aorta, the left T8 and T9 intercostal arteries are exposed and clamped from outside the aorta (Figure 6).
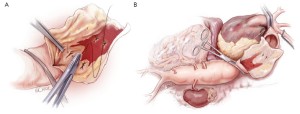
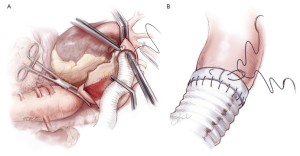
Five minutes after aortic clamping and observing the MEPs for decreased signaling, the aorta is opened. Back bleeding from the right intercostal arteries is controlled by inserting 2-Fr balloon-tipped catheters into the respective ostia (Figure 7), in order to prevent spinal cord ischemia from a steal phenomenon. Patent orifices of intercostal arteries (no more than two pairs) are then anastomosed to a side hole of the graft as an aortic patch using an inclusion technique with a 4-0 monofilament suture (17-mm needle) (Figure 8A). Another technique to reattach the intercostal arteries is through a graft interposition, where several small grafts connect to the orifices of the intercostal arteries (Figure 8B). This is followed by transposing the graft clamp distally to allow perfusion of the newly anastamosed intercostal arteries (Figure 9). Additional neighboring intercostal arteries are reconstructed and reperfused in the same fashion according to the findings of the preoperative CT angiogram.
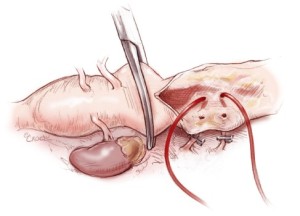
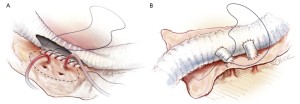
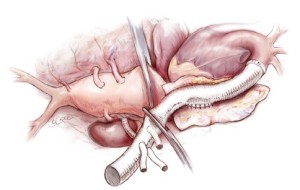
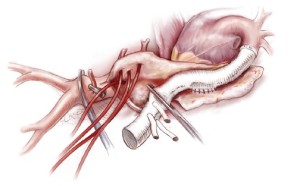
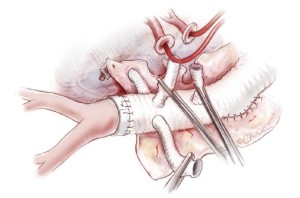
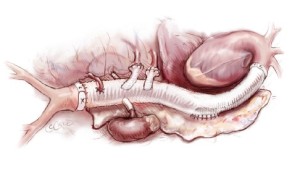
After reconstruction of the intercostal arteries, 30 mg of Edaravone is routinely administrated to prevent neurologic injury. The infra-renal portion of the abdominal aorta is then clamped and opened. The back-bleeding from lumbar arteries is controlled similarly to intercostal back-bleeding. The 4 visceral arteries are perfused using 8-Fr size balloon-tipped catheters (Fuji phycon®, Tokyo) via a single roller pump at arterial flows of 150-200 mL/min (Figure 10). Upon completion of visceral artery cannulation, rewarming is initiated. Each visceral artery is individually dissected to make a button (Figure 11). First, the right renal artery is anastomosed to one side branch of the graft using a 4-0 monofilament suture. Then, the distal anastomosis between the graft and the aorta is performed using a 4-0 suture (22-mm needle) and reinforced with Teflon felt. After this is completed, the buttons of the celiac, superior mesenteric artery and left renal artery are anastomosed to side branches with 4-0 sutures on a 17-mm needle. The inferior mesenteric artery is often reattached directly to an opening in the graft using a 5-0 monofilament suture with a 17-mm needle. After completion of all anastamoses, the patient is weaned from cardiopulmonary bypass and hemostasis is achieved after protamine is administered (Figure 12).
Results
Nine (5.9%) patients died within 30 days [Crawford classification I =1 (4.8%), II =1 (2.3%), III =6 (8.2%), IV =1 (6.7%)], while hospital mortality occurred in 20 (13.2%) patients [Crawford extent I =3 (14.3%), II =2 (4.7%), III =13 (17.8%), IV =2 (13.3%)]. In emergent and urgent cases, 30-day and hospital mortality rates were 20% and 32%, respectively. Independent risk factors for hospital mortality were emergency surgery (OR 13.4, P=0.003) and aortic cross clamping >2 hours (OR 5.7, P=0.04). Postoperative spinal cord ischemic complications in 16 (10.5%) patients; 8 patients developed paraplegia and 8 had paraparesis [2 (9.5%) in Crawford extent I, 3 (7.0%) in II, 11 (15.1%) in III, and zero in IV]. In emergent and urgent cases, spinal cord ischemic complications developed in 12.0%. Risk factors for spinal cord ischemia were prior surgery involving the descending thoracic or abdominal aorta (OR 3.75, P=0.05), diabetes mellitus (OR 5.49, P=0.03) and post-bypass hypotension <80 mmHg (OR 1.06, P=0.03). Postoperative survival at 5 years for all patients was 83.6±4.5%. 5-year survival was 47.5±8.6% in patients with spinal cord ischemic complications and was 88.9±10.4% in patients without these complications. Freedom from aortic events, such as redo operation, rupture of residual aneurysm, and false aneurysm formation, was 91.8±4.3% at 5 years.
Comments
Although the usefulness of extracorporeal circulation during reconstruction of the thoracoabdominal aorta is well documented, left heart bypass with a reduced dose of heparinization (1,2) and repair without cardiopulmonary bypass altogether (3,4) have been shown to produce acceptable surgical outcomes. At our institution, we have primarily instituted cardiopulmonary bypass instead of either of these two alternatives as it provides stable hemodynamic support if pulmonary function deteriorates. An additional advantage of this technique is that a cardiotomy suction can be used in case of unexpected massive bleeding. Lastly, the benefits of hypothermia on end-organ protection can be taken advantage of as the patient’s body temperature is easily controlled through core-cooling during the bypass period (5,6).
Revascularization of the intercostal arteries represents an important technical step during repair of thoracoabdominal aneurysms. Several surgical techniques have been described, the most simple of which is an aortic patch anastomosis to the prosthetic graft. The main advantage of the patch technique is the reduced number of anastomoses (1). However, isolated reimplantation of each intercostal artery might be necessary for larger aneurysms, particularly if the ostia of the intercostal arteries are widely displaced from each other. Moreover, it has been reported that when a segment of aortic wall is left in situ to facilitate branch reconstruction, it may become aneurysmal, particularly in patients with Marfan syndrome (7). Dardik et al. (8) reported a prevalence of patch aneurysm of 7.5% in 107 patients who underwent thoracoabdominal aneurysm repair. Lombardi et al. (9) reported 3 patients with patch aneurysm among 20 patients who required reoperation after previous thoracoabdominal aneurysm repair. Kouchoukos et al. (10) reported 2 cases of patch aneurysm in Marfan patients.
The major drawback of the graft interposition technique is suboptimal patency of the reconstructed grafts when compared with the patch technique, where almost all attached intercostal arteries should remain patent. In our series, early patency of the interpositioned grafts was only 70% by angiography or CT scan. Moreover, more than half of the grafts were occluded in patients who had postoperative paraplegia (11).
Other adjuncts for spinal cord protection played important roles in the surgical outcomes of this cohort. The advent of the MR and CT imaging has facilitated identification of the great radicular artery, the artery of Adamkiewicz. Yamada et al. (12) firstly demonstrated the Adamkiewicz artery in the majority of the patients with descending or thoracoabdominal aortic aneurysms. Yoshioka et al. (13) demonstrated several intercommunicating collateral pathways between the critical intercostal arteries. These non-invasive imaging techniques have simplified the surgical procedure to reconstruct the critical intercostal arteries and neighboring ones. Additionally, concomitant use of intraoperative transcranial-stimulated MEPs of the spinal cord enables detection of cord ischemia and identifies critical intercostal arteries in real time (14). Furthermore, CSFD alleviates secondary compression of the spinal cord due to ischemic edema (15).
Regarding techniques for reconstructing visceral arteries, Carrel et al. (16) reported their technique of separate revascularization of the visceral arteries in thoracoabdominal aneurysm repair. Similarly, Safi et al. (17) reported their use of a presewn 4-branched graft for visceral artery reconstruction during thoracoabdominal aortic repair using a deep hypothermic technique. Visceral protection has been achieved by separate visceral perfusion with graft flows between 100-200 mL/min. Some reports (18,19) state that systemic deep hypothermia provides excellent organ protection; however, deep hypothermia also has potential disadvantages including a longer cardiopulmonary bypass time, increased coagulopathy (20), and increased lung dysfunction (21). On the other hand, Coselli et al. (22) emphasized the superiority of infusing cold crystalloid solution into the renal arteries. We favor, however, perfusing viscera rather than infusing cold saline or no perfusion when the aortic cross clamp time is prolonged. We believe that each button anastomosis facilitates mobilization of the branches that may improve the postoperative patency rate of visceral arterial anastomoses when compared with the patency rate following the inclusion technique. Care must be taken to avoid rotation of the viscera to the right during reconstruction becasue the visceral arteries, especially the left renal artery, may kink after subsequent repositioning of the viscera.
The importance of postoperative pain control cannot be overemphasized when patients start respiratory and ambulatory rehabilitation. In such circumstances, paravertebral nerve blockade enables physiotherapy even in the elderly (23).
Conclusions
Early results of patients who underwent surgery for thoracoabdominal aortic aneurysms, with reconstruction of the intercostal arteries and using a branched replacement graft, was satisfactory.
Acknowledgements
We thank Ms. Beth Croce, Illustration Editor, and Dr. Ashutosh Hardikar, Art of Operative Techniques Section Editor, for making these highly detailed illustrations.
Disclosure: The authors declare no conflict of interest.
References
- Crawford ES, Crawford JL, Stowe CL, et al. Total aortic replacement for chronic aortic dissection occurring in patients with and without Marfan’s syndrome. Ann Surg 1984;199:358-62. [PubMed]
- Hollier LH, Symmonds JB, Pairolero PC, et al. Thoracoabdominal aortic aneurysm repair. Analysis of postoperative morbidity. Arch Surg 1988;123:871-5. [PubMed]
- Cambria RP, Davison JK, Zannetti S, et al. Thoracoabdominal aneurysm repair: perspectives over a decade with the clamp-and-sew technique. Ann Surg 1997;226:294-303; discussion 303-5. [PubMed]
- Tefera G, Acher CW, Wynn MM. Clamp and sew techniques in thoracoabdominal aortic surgery using naloxone and CSF drainage. Semin Vasc Surg 2000;13:325-30. [PubMed]
- Okita Y, Takamoto S, Ando M, et al. Repair for aneurysms of the entire descending thoracic aorta or thoracoabdominal aorta using a deep hypothermia. Eur J Cardiothorac Surg 1997;12:120-6. [PubMed]
- von Segesser LK, Marty B, Mueller X, et al. Active cooling during open repair of thoraco-abdominal aortic aneurysms improves outcome. Eur J Cardiothorac Surg 2001;19:411-5; discussion 415-6. [PubMed]
- Ingu A, Ando M, Okita Y, et al. Redo operation for thoracoaortic aneurysm after entire aortic replacement. Ann Thorac Surg 2001;72:1766-7. [PubMed]
- Dardik A, Perler BA, Roseborough GS, et al. Aneurysmal expansion of the visceral patch after thoracoabdominal aortic replacement: an argument for limiting patch size? J Vasc Surg 2001;34:405-9; discussion 410. [PubMed]
- Lombardi JV, Carpenter JP, Pochettino A, et al. Thoracoabdominal aortic aneurysm repair after prior aortic surgery. J Vasc Surg 2003;38:1185-90. [PubMed]
- Kouchoukos NT, Masetti P, Castner CF. Use of presewn multiple branched graft in thoracoabdominal aortic aneurysm repair. J Am Coll Surg 2005;201:646-9. [PubMed]
- Omura A, Tanaka A, Miyahara S, et al. Early and late results of graft replacement for dissecting aneurysm of thoracoabdominal aorta in patients with Marfan syndrome. Ann Thorac Surg 2012;94:759-65. [PubMed]
- Yamada N, Okita Y, Minatoya K, et al. Preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography in patients with descending or thoracoabdominal aortic aneurysms. Eur J Cardiothorac Surg 2000;18:104-11. [PubMed]
- Yoshioka K, Niinuma H, Ohira A, et al. Three-dimensional demonstration of the artery of Adamkiewicz by multidetector-row computed tomography. Ann Thorac Surg 2004;78:719. [PubMed]
- de Haan P, Kalkman CJ. Spinal cord monitoring: somatosensory- and motor-evoked potentials. Anesthesiol Clin North America 2001;19:923-45. [PubMed]
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [PubMed]
- Carrel TP, Signer C. Separate revascularization of the visceral arteries in thoracoabdominal aneurysm repair. Ann Thorac Surg 1999;68:573-5. [PubMed]
- De Rango P, Estrera AL, Miller C 3rd, et al. Operative outcomes using a side-branched thoracoabdominal aortic graft (STAG) for thoraco-abdominal aortic repair. Eur J Vasc Endovasc Surg 2011;41:41-7. [PubMed]
- Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2011;141:953-60. [PubMed]
- Fehrenbacher JW, Siderys H, Terry C, et al. Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg 2010;140:S154-60; discussion S185-90.
- Westaby S. Coagulation disturbance in profound hypothermia: the influence of anti-fibrinolytic therapy. Semin Thorac Cardiovasc Surg 1997;9:246-56. [PubMed]
- Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47-55. [PubMed]
- LeMaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19. [PubMed]
- Català E, Casas JI, Unzueta MC, et al. Continuous infusion is superior to bolus doses with thoracic paravertebral blocks after thoracotomies. J Cardiothorac Vasc Anesth 1996;10:586-8. [PubMed]





