Open surgical repair of thoracoabdominal aneurysms - the Massachusetts General Hospital experience
Introduction
The modern era of surgical management of thoracoabdominal aneurysms (TAA) began with the pioneering work of E. Stanley Crawford (1); this benchmark series reported an operative mortality of 10% with an incidence of spinal cord ischemia (SCI) of 16%, some 50% of which were total paraplegia. Over a 20 year period until 2006, TAA repair at our institution (2) was predominantly performed using a simplified clamp and sew approach in accordance with Dr. Crawford’s teachings which emphasized operative expediency and technical efficiency (2). Management of TAA during this time was typically performed without use of distal perfusion techniques. Adjunct use included routine cerebrospinal fluid (CSF) drainage (3,4), aggressive intercostal re-implantation (3,5,6), regional hypothermia for spinal cord protection (7,8), infusion of hypothermic renal preservation fluid for prevention of renal failure, and in-line mesenteric shunting (9) to reduce complications resulting from visceral ischemia. Using this approach we achieved favorable results with an overall operative mortality of 8% (6% elective and 13% urgent) with significant SCI in 8% of patients (2).
Largely driven by the apparent failure of epidural cooling to drive SCI to less than 5%, we modified operative management of TAA in recent years in an effort to further reduce spinal cord complications. As our experience has shown (10) that patients with type IV TAA can safely be managed with a simplified clamp/sew approach with favorable results, this evolution in operative strategy has been exclusively implemented in management of patients with extents I-III TAA. In essence the rationale exploited the collateral network concept (11-13): we adopted routine use of distal aortic perfusion (DAP) via left atrial to femoral bypass to support the collateral circulation to the spinal cord during the period of aortic cross clamp application. Additionally, intra-operative motor evoked potential (MEP) monitoring (14,15) has afforded the ability to dynamically assess spinal cord ischemia during aneurysm reconstruction. As a result, we have abandoned aggressive intercostal re-implantation in favor of a selective MEP driven re-implantation strategy. Clinical outcomes resulting from this evolution in operative strategy for extents I-III TAA have recently been published and are further highlighted herein (16).
Methods
Data for all thoracic aortic procedures performed at Massachusetts General Hospital (MGH) are prospectively entered into our institutional Thoracic Aortic Center (TAC) database. TAC data is independently verified and validated by hospital employed clinical nurses who maintain data integrity. Using the TAC dataset, we performed a retrospective analysis of all consecutive types I-III repairs performed at the MGH from September 1989 through December 2009. Prior to July 2006, extents I-III TAA were repaired with a predominantly clamp and sew approach with the aforementioned protective adjuncts utilized. During this era distal aortic perfusion was seldom utilized (~10%), except in patients with significant renal dysfunction or those in whom the proximal reconstruction was anticipated to be technically challenging. Intercostal re-implantation within the critical T9-L1 region, when technically feasible, was routinely performed in accordance with both published literature, and our own observations that sacrifice of T9-L1 intercostal vessels was a correlate of SCI (5,6). Detailed descriptions of the technical conduct of TAA repair (clamp/sew and DAP/MEP) have been published elsewhere (7,16).
The impact of demographic factors and clinical features on peri-operative outcomes were studied. Chronic renal insufficiency (CRI) was defined as a baseline serum creatinine greater than 1.5 mg/dL. Coronary artery disease (CAD) was defined as a history of myocardial infarction, positive cardiac stress test, or previous percutaneous or open surgical coronary artery revascularization. Pulmonary disease was determined by pre-operative pulmonary function testing in the majority of patients. Aneurysm related features included aneurysm type, pathology, and urgent vs. elective repair. An urgent operation was defined as symptomatic presentation necessitating Intensive care unit (ICU) admission for invasive hemodynamic monitoring and operative reconstruction within 48 hours of admission.
Statistical analysis
All demographic data and clinical features are presented as percent prevalence in the study population. All mean data are presented as mean ± standard deviation (SD). Statistical analysis was performed by using two-tailed t tests for continuous variables and chi-square analysis for categorical data. Multivariable regression analysis was performed to identify predictors of the composite endpoint of death and spinal cord ischemia. Results with a P<0.05 were considered statistically significant. Statistical analysis was performed using SAS version 9.2 (Cary, NC).
Results
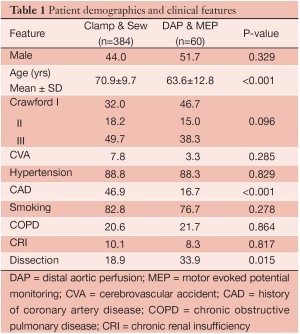
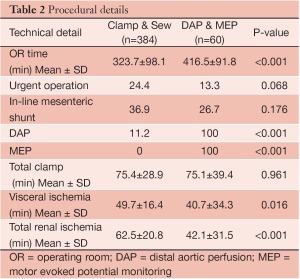
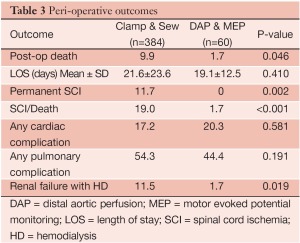
Four hundred and forty-four patients underwent extents I-III TAA repair over the study period, including 384 patients treated with clamp/sew and 60 patients with the modified operative strategy using DAP/MEP. Clinical and demographic features are summarized in Table 1. Patients treated with DAP/MEP were younger and more likely to harbor aneurysms of chronic dissection etiology. Technical features of operative conduct are presented in Table 2. Patients treated with DAP/MEP were more likely to have lower visceral ischemia and total renal ischemia times, despite having have longer operative times. Peri-operative outcomes are summarized in Table 3. Operative morality (30-day), permanent SCI and the composite endpoint of permanent SCI and death were all significantly lower in patients treated with DAP/MEP. On multivariable logistic regression modeling, DAP/MEP use was independently associated with a significant reduction in the composite outcome of perioperative SCI/death (OR 0.1, 95% CI: 0.012-0.67; P=0.01).
Full table
Full table
Full table
Discussion
Thoracoabdominal aortic (TAA) aneurysm repair has historically been associated with significant morbidity and mortality (1), in particular in administrative database studies; however recent large, single center reports have presented markedly improved results most notably with respect to SCI (17-21). This improvement in outcomes reflects the impact of cord protection strategies adopted over time (22). On the contrary, patients undergoing extent IV TAA, repair have uniformly had a very low risk of SCI independent of variations in operative technique or adjunct use (18,23-26). Our stance on operative management of extent IV TAA has continued to emphasize operative expediency and efficiency, using a standardized clamp/sew approach. Using this approach we have achieved favorable results in the management of extent IV TAA with an operative mortality rate of 2.8% in a recently published series of 179 repairs, more than 90% of which were repaired using a standardized technique (10). Our results were also notable for a favorable SCI rate of 2.2% without routine use of cord protective strategies such as CSF drain (<15%) or epidural cooling (12%). Regression analysis of the data from that series showed that technical factors in aneurysm reconstruction and protective adjuncts were not predictive for development of SCI, strongly suggesting that a continued clamp/sew approach in patients with extent IV TAA is appropriate.
Finite improvements in SCI and mortality following repair of more complex TAA in our hands prompted an evolution in operative strategies for management of extent I-III TAA. Our current understanding was predominantly influenced by the concept of the spinal cord’s collateral network as originally described by Griepp et al. (11,12). Recent magnetic resonance angiography studies have elegantly demonstrated the existence of a robust network of collateral vessels which reconstitute the great radicular artery via intersegmental collaterals emanating from distal segmental arteries (many vertebral levels away) and even more distal pelvic and hypogastric branches (13). The existence of such collateral vessels argues in favor of assisted circulation techniques to support perfusion of these vessels intra-operatively during cross clamp application. Clinical and physiological support for this has been provided using intra-operative monitoring to detect spinal cord ischemia. Jacobs et al. clinically observed that in patients with complete occlusion of critical and distal intercostal vessels, MEPs were highly dependent upon maintenance of pelvic perfusion using atrial-femoral bypass with rapid deterioration in MEP amplitudes with temporary discontinuation of the bypass circuit during distal clamp application (17,27). Further clinical support for use of distal perfusion has been provided by Safi et al. who have reported on the reduction of SCI and mortality following institution of a combined adjuncts comprised of CSF drainage, passive hypothermia (32-34 ℃) and distal aortic perfusion (28). Using this adjunct combination these investigators effectively reduced overall SCI rates to 3.3%, with the majority of benefit derived from reduction of SCI in TAA types I and II from 31% to 9% (29).
The current review highlights progressively improved outcomes coincident with the evolution in operative strategy for extents I-III TAA. Although the benefit of an expedient operation seems intuitively logical, these data show that we have achieved significant improvement in operative mortality and SCI (1.7% combined) despite longer operating times, higher blood turnover, use of sequential aortic cross clamp application, and limited (~10%) intercostal re-implantation (16). Our comparative results suggest that our current techniques utilizing DAP to support the spinal cord’s collateral network and MEP monitoring to dictate selective intercostal re-implantation is the favored approach in treating patients with extents I-III TAA.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68.
- Conrad MF, Crawford RS, Davison JK, et al. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg 2007;83:S856-S861.
- Coselli JS, LeMaire SA, Koksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9.
- Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg 2004;40:36-44.
- Safi HJ, Miller CC 3rd, Carr C, et al. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg 1998;27:58-66.
- Cambria RP, Davison JK, Carter C, et al. Epidural cooling for spinal cord protection during thoracoabdominal aneurysm repair: a five-year experience. J Vasc Surg 2000;31:1093-102.
- Davison JK, Cambria R, Vierra D, et al. Epidural cooling for regional spinal cord hypothermia during thoracoabdominal aneurysm repair. J Vasc Surg 1994;20:304-10.
- Cambria RP, Clouse WD, Davison JK, et al. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 2002;236:471-9.
- Cambria RP, Davison JK, Giglia JS, et al. Mesenteric shunting decreases visceral ischemia during thoracoabdominal aneurysm repair. J Vasc Surg 1998;27:745-9.
- Patel VI, Ergul E, Conrad MF, et al. Continued favorable outcomes of type IV thoracoabdominal aortic aneurysm repair. J Vasc Surg 2011;53:1492-8.
- Etz CD, Halstead JC, Spielvogel D, et al. Thoracic and thoracoabdominal aneurysm repair: is reimplantation of spinal cord arteries a waste of time? Ann Thorac Surg 2006;82:1670-7.
- Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg 2007;83:S865-9.
- Backes WH, Nijenhuis RJ, Mess WH, et al. MRA of collateral blood supply to the spinal cord in thoracic and thoracoabdominal aortic aneurysm patients. J Vasc Surg 2008;48:261-71.
- de Haan P, Kalkman CJ, de Mol BA, et al. Efficacy of transcranial motor evoked myogenic potentials to detect spinal cord ischemia during operations for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 1997;113:87-100.
- Jacobs MJ, Mess W, Mochtar B, et al. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J Vasc Surg 2006;43:239-46.
- Conrad MF, Ergul EA, Patel VI, et al. Evolution of operative strategies in open thoracoabdominal aneurysm repair. J Vasc Surg 2011;53:1195-201.
- Jacobs MJ, de Mol BA, Elenbaas T, et al. Spinal cord blood supply in patients with thoracoabdominal aortic aneurysms. J Vasc Surg 2002;35:30-7.
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4.
- Safi HJ, Estrera AL, Azizzadeh A, et al. Progress and future challenges in thoracoabdominal aortic aneurysm management. World J Surg 2008;32:355-60.
- Acher CW, Wynn MM, Mell MW, et al. A quantitative assessment of the impact of intercostal artery re-implantation on paralysis risk in thoracoabdominal aortic aneurysm repair. Ann Surg 2008;248:529-40.
- Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5.
- Acher CW, Wynn M. A modern theory of paraplegia in the treatment of aneurysms of the thoracoabdominal aorta: an analysis of technique specific observed/expected ratios for paralysis. J Vasc Surg 2009;49:1117-24.
- Kieffer E, Laurent C, Godet G, et al. Type IV thoracoabdominal aneurysm repair: predictors of postoperative mortality, spinal cord injury, and acute intestinal ischemia. Ann Vasc Surg 2008;22:822-28.
- Achouh PE, Estrera AL, Miller CC 3rd, et al. Role of somatosensory evoked potentials in predicting outcomes during thoracoabdominal aortic repair. Ann Thor Surg 2007;84:782-7; discussion 787-8.
- Schwartz LB, Belkin M, Donaldson MC, et al. Improvement in results of repair of type IV thoracoabdominal aortic aneurysms. J Vasc Surg 1996;24:74-81.
- Richards JM, Nimma JF, Moores CR, et al. Contemporary results for open repair of suprarenal and type IV thoracoabdominal aortic aneurysms. B J Surgery 2010;97:45-9.
- Nijenhuis RJ, Jacobs MJ, Schurink GW, et al. Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg 2007;45:71-7.
- Safi HJ, Miller CC 3rd, Huynh TT, et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg 2003;238:372-80.
- Safi HJ, Estrera AL, Miller CC, et al. Evolution of risk for neurological deficit after descending and thoracoabdominal aortic repair. Ann Thor Surg 2005;80;2173-9.





