Minimally invasive reoperative aortic valve replacement
Introduction
Increasing life expectancy, coupled with an aging population, has created an increased need for cardiovascular disease treatment in very elderly patients and patients who have already undergone to cardiac surgery. The frequent use of biological prostheses may be an additional factor in the growing number of aortic valve reoperations. This creates problems, given the need to reduce healthcare expenses through shorter hospital stays, fewer blood transfusions, and better postoperative outcomes by avoiding prolonged ventilation time and wound infections. To reach these goals, minimally invasive approaches have been used since 1996 in cases of reoperative aortic valve replacement (rAVR) (1-3). Minimally invasive surgery can only be introduced only after proving that it is at least as safe and efficient as the gold standard full sternotomy (4).
Initial data regarding the use of upper “J” hemi-sternotomy in rAVR were encouraging, showing a reduction in the biological trauma. Many advantages were reported for this technique, especially in cases of patent bypass grafts (5,6). This was primarily attributable to the lesser dissection of mediastinal tissues that avoids excessive blood losses. For this reason, some literature suggests that this approach should only be adopted in high-risk patients (7,8).
Key aspects in redo aortic valve replacement
Aortic valve replacement (AVR) through an upper “J” sternotomy approach has been widely described in literature. Whilst more complex, minimally invasive, reoperative approaches exist, they are only being performed in a few centers (5-8).
In the current era, the benchmark for rAVR is the full sternotomy, often associated with peripheral cannulation (9,10). The advantages and disadvantages of minimally invasive techniques when compared with the conventional approach are still not well established, with reported concerns regarding technical difficulties as well as myocardial protection issues. The relative merits of minimally invasive rAVR are discussed in this report.
Surgical approach
The minimally invasive surgical option should be considered because of various advantages. Firstly, the mini-sternotomy approach reduces surgical trauma by reducing the area of pericardiolysis since mediastinal dissection is limited to the ascending aorta and a small portion of the right atrium for the purpose of cannulation. The right ventricle does not need to be dissected, which lowers the risk of injury. The result is a decrease of debridement and bleeding, especially in cases of patent bypasses (11,12).
Although the operative field is smaller, the upper J mini-sternotomy guarantees an optimal surgical exposure and a proper aortic valve exposure. However, an alternative to upper J hemi-sternotomy may be right mini-thoracotomy (12). We believe that this approach is ideal in cases of primary isolated AVR but will be more technically challenging in reoperative cases due to adhesions of the heart to surrounding tissues caused by the previous surgery. It is obvious that the hemi-sternotomy has a better cosmetic result too, avoiding an additional scar. Our favored approach is an upper “J” shaped or “linear” re-hemisternotomy performed down to the 4th right intercostal space through a 6 cm skin incision (Figure 1).
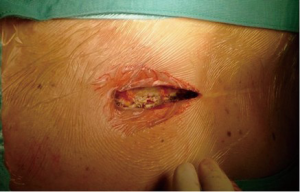
Surgical dissection is mainly carried out towards the right side of the mediastinum. A minimal dissection around the aorta and the right atrium is also performed to allow cannulation for cardiopulmonary bypass and aortic cross-clamp placement.
Cannulation site
Is peripheral cannulation essential in cases of reoperative surgery? While there is evidence suggesting that the exposition of the femoral vessel (or alternatively the axillary artery) for cardiopulmonary bypass access is the safer approach, it is not free from potential complications (3,10). Whenever possible, we prefer to adopt a central cannulation, since this approach is considered to resemble normal physiology more closely. Furthermore, should there be a need for femoral vessel isolation, this can be rapidly obtained. Since 2010, cardiopulmonary bypass was obtained through central cannulation in all patients treated-only in two patients a femoral arterial cannulation was necessary. Recently, other Authors with excellent experience agree with the utilization of central cannulation whenever it is possible (7,8).
Myocardial protection
The myocardial protection is of critical importance, especially in cases of previous bypass operation with patent left internal mammary artery (LIMA). The classical strategy involves dissection and temporary occlusion of the LIMA pedicle during cardioplegic arrest to avoid myocardial regional warming and cardioplegic solution “washout”. This technique carries the risk of graft injury and inadequate myocardial protection with consequent myocardial infarction (13). We propose to maintain the LIMA unclamped and to adopt a blood normothermic antegrade cardioplegia (since 2013, a hypothermic blood cardioplegia with procaine has been preferred). An initial dose of cardioplegia is needed to arrest the heart in order to open the aorta and explore the aortic valve; then the heart usually restarts to contract, sometimes showing a short period of ventricular fibrillation. In this case, we administer an additional shot of cardioplegia directly in the coronary ostia every 20 minutes, however the main goal is to keep the left heart well vented without cooling down the patient. Our results, in addition to data from the literature, do not show an increased incidence of postoperative malperfusion syndrome (6,14-16). During cardioplegic arrest we use 3-0 prolene running sutures to implant the valve prostheses (Figure 2). With this technique it is possible to significantly shorten the aortic cross-clamp time and further minimize the incidence of perioperative myocardial infarction. In our experience, which currently involves 52 patients, the incidence of peri-operative acute myocardial infarction (defined as a new Q-wave in the electrocardiogram or a new wall motion abnormality on echocardiography) was zero. This cohort included patients presenting with patent LIMA-RIMA Y graft (total heart perfusion). This observation supports our satisfactory results to date (14). Other authors reported no increased risk in perioperative morbidity and mortality in the case of an unclamped LIMA graft (6,16), with other strategies been proposed. In 1999, Byrne and colleagues (11) reported their experience of leaving the LIMA graft unclamped while performing the AVR under hypothermia at 20 °C and cardioplegic arrest. Kaneko’s proposal is to cool the patients from 25° to 30° and to adopt both antegrade and retrograde cardioplegia. When cardiac activity was observed because of a patent LIMA graft, additional systemic potassium was given through the pump (40 mEq) to a dosage of 6.0 to 7.0 mEq (7,8).
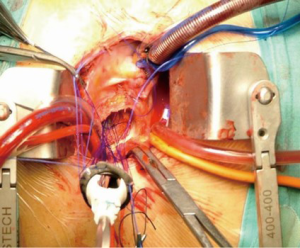
Adequate deairing
The left ventricle is vented routinely through the right upper pulmonary vein to achieve an adequate deairing. In this kind of surgery, the left ventricular vent is mandatory also to avoid ventricular dilatation. In addition, continuous carbon dioxide is used, and adequate deairing is monitored by transesophageal echocardiography. With the patient in the Trendelenburg position, the aorta is unclamped and the ascending aorta vent turned on. This, of course, it is the standard approach adopted in minimally invasive surgery (6-8).
Pacing wires
A potential inconvenience, especially in the cases of a small incision, may arise when placing ventricular pacing wires. If this appears to be challenging due to the adherences and the small surgical field, we position temporary endocavitary pacing leads through the right internal jugular vein. The same solution has already been reported previously (6-8).
Our experience
In the last seven years, 52 adult patients underwent reoperative isolated AVR through a minimally invasive approach. The outcomes of patients (in-hospital mortality about 1.9%) are encouraging and intraoperative times are highly competitive with published data on the standard full sternotomy (Table 1). Our results confirm that a small incision is associated with less postoperative pain and better respiratory function, as supported by existing literature (14,17-21).
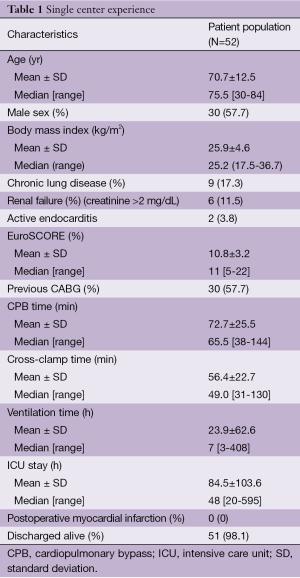
Full table
Conclusions
In conclusion, our experience supports existing literature in suggesting the beneficial effects of a minimally invasive approach to AVR. Indeed, successful outcomes can be achieved without compromising the efficacy of the entire operative procedure. This approach may also be used in reoperative surgery (7). The minimally invasive surgery approach reduces biological aggressiveness, which may translate into shorter operation duration, and lower risk of sternal instability or infection. All these elements contribute to a lower operative morbidity and mortality. Our current results and the recent evidence in the literature (4,7,14,17-21) are encouraging, warranting the need for more randomized prospective studies in the future.
Acknowledgements
We gratefully acknowledge the assistance of Professor Luigi Tavazzi and Maria Cristina Jori, M.D. in helping us with the statistical analyses and editorial support.
Disclosure: The authors declare no conflict of interest.
References
- Tam RK, Garlick RB, Almeida AA. Minimally invasive redo aortic valve replacement. J Thorac Cardiovasc Surg 1997;114:682-3. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Pineda AM, Santana O, Lamas GA, et al. Is a minimally invasive approach for re-operative aortic valve replacement superior to standard full resternotomy? Interact Cardiovasc Thorac Surg 2012;15:248-52. [PubMed]
- Byrne JG, Karavas AN, Filsoufi F, et al. Aortic valve surgery after previous coronary artery bypass grafting with functioning internal mammary artery grafts. Ann Thorac Surg 2002;73:779-84. [PubMed]
- Gaeta R, Lentini S, Raffa G, et al. Aortic valve replacement by ministernotomy in redo patients with previous left internal mammary artery patent grafts. Ann Thorac Cardiovasc Surg 2010;16:181-6. [PubMed]
- Kaneko T, Leacche M, Byrne J, et al. Reoperative minimal access aortic valve replacement. J Thorac Dis 2013;5:S669-72. [PubMed]
- Kaneko T, Loberman D, Gosev I, et al. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155-62. [PubMed]
- Onorati F, Biancari F, De Feo M, et al. Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative†. Eur J Cardiothorac Surg 2015;47:269-80. [PubMed]
- Ruttmann E, Gilhofer TS, Ulmer H, et al. Propensity score-matched analysis of aortic valve replacement by mini-thoracotomy. J Heart Valve Dis 2010;19:606-14. [PubMed]
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [PubMed]
- Pineda AM, Santana O, Reyna J, et al. Outcomes of reoperative aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis 2013;22:50-5. [PubMed]
- Gillinov AM, Casselman FP, Lytle BW, et al. Injury to a patent left internal thoracic artery graft at coronary reoperation. Ann Thorac Surg 1999;67:382-6. [PubMed]
- Mikus E, Calvi S, Tripodi A, et al. Upper ‘J’ ministernotomy versus full sternotomy: an easier approach for aortic valve reoperation. J Heart Valve Dis 2013;22:295-300. [PubMed]
- Dell’Amore A, Del Giglio M, Calvi S, et al. Mini re-sternotomy for aortic valve replacement in patients with patent coronary bypass grafts. Interact Cardiovasc Thorac Surg 2009;9:94-7. [PubMed]
- Smith RL, Ellman PI, Thompson PW, et al. Do you need to clamp a patent left internal thoracic artery-left anterior descending graft in reoperative cardiac surgery? Ann Thorac Surg 2009;87:742-7. [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A Meta-Analysis of Minimally Invasive Versus Conventional Sternotomy for Aortic Valve Replacement. Ann Thorac Surg 2014;98:1499-1511. [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5.
- Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25.
- Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460-5; discussion 465-6. [PubMed]





