Minimally invasive aortic valve surgery: Cleveland Clinic experience
Introduction
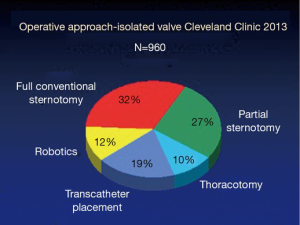
Since the minimally invasive approach to valve surgery was first brought to the Cleveland Clinic by Cosgrove, it has been increasingly adopted by cardiac surgeons worldwide (1,2). Minimally invasive surgery has evolved to become the standard of care for isolated aortic or mitral valve disease at our institution, with a wide variety of approaches, techniques and cannulation strategies employed over the past two decades (3,4) (Figure 1). Techniques have been refined and iterative improvements have continued to allow for expanded indications and improved outcomes.
While minimally invasive approaches to aortic and mitral valve surgery have evolved in parallel, there are unique considerations that inform the choice of incision for such patients. In the case of degenerative mitral valve disease, robotic and thoracotomy approaches have become the norm, while the protocol for aortic valve disease is more complex. The combination of disease state, concomitant cardiac disease, age, comorbid conditions, and procedure type define a different paradigm for decision making in aortic valve disease.
The primary disease process for which patients are referred for aortic valve surgery remains aortic stenosis. This population is older and more likely to have concomitant vascular disease compared to the mitral valve population. In addition to senile aortic stenosis, bicuspid aortic valve disease is a major etiology referred for surgery. These patients present a unique challenge in tailoring the operation to the individual, as both the treatment of associated aneurysm and prevention of future disease or need for reoperation must be considered.
The objectives of this study are to describe the trends in minimally invasive aortic valve surgery (MIAVS) at the Cleveland Clinic from the inception of this technique to the present; to review the current practice of MIAVS in terms of patient selection, known pitfalls, cannulation and protection strategies; and to review outcomes of this current strategy.
Methods
Patients
From 1996 to 2013, 22,766 patients underwent aortic valve operations (including reoperations and multi-component procedures) at the Cleveland Clinic. Of these, 3,385 (15%) have been performed with a minimally invasive approach. All patients undergoing cardiac surgery at the Cleveland Clinic are entered into the Cardiovascular Information Registry (CVIR), which includes a collection of preoperative demographic and comorbidity data, indications for surgery, operative variables, in-hospital complications, and operative mortality. Routine telephone follow-up is available for those patients with a surgery date before 2012. Survival data has been supplemented with Social Security Death Index data. All aortic valve operations regardless of type were included in the cohort for analysis.
Interventions
Incision selection for valve surgery has been at the discretion of the surgeon without a formal algorithmic approach. In the early years after the introduction of MIAVS to the Cleveland Clinic, minimally invasive surgery was largely performed by a small subgroup of surgeons. However, recent trends show surgeons utilizing minimally invasive approaches for isolated aortic valve disease. Patient outcomes for standard and minimally invasive surgery are reviewed on a regular basis at monthly quality staff meetings. All mortalities undergo a formal presentation and review by the staff. Trends in major morbidity are reviewed in detail and have led to continuous refinement of surgical techniques, perfusion, and protection strategies.
For patients requiring isolated valve surgery at the Cleveland Clinic, minimally invasive approaches dominate and the specific incision is tailored to the patient based on the valve involved, morphology of disease, patient-specific anatomy, and surgeon preference (Figure 2). Most patients with a primary indication for aortic valve surgery are amenable to a MIAVS approach, so a description of contraindications or relative contraindications is worthwhile. The need for coronary revascularization is usually a contraindication to MIAVS, although some patients have undergone hybrid aortic valve replacement (AVR) plus percutaneous coronary stenting, while several others have had right coronary bypass grafting at the time of MIAVS. Emergency operations for endocarditis or acute proximal aortic dissection are routinely performed through a full sternotomy. Although reoperations have been performed using MIAVS techniques, we have considered it a relative contraindication in recent years, given the potential risks of less than ideal cardiac protection in relation to potential benefits. Finally, although aortic root replacement is frequently performed through a mini incision, when the patient is planned for a modified David’s reimplantation procedure or Ross procedure, we have preferred the exposure provided by a full sternotomy approach.
All other patients, including those requiring aortic replacement with or without circulatory arrest, multi-valve operations, and even those with atrial fibrillation or severe comorbidities, are candidates for a MIAVS approach (5,6).
Standard preoperative cardiac workup has included plain chest radiography, coronary angiography, and routine laboratory studies in addition to echocardiography. Cardiac computed tomography is obtained selectively, usually for patients with suspicion of concomitant aortic disease or those being considered for right anterior thoracotomy (7).
Surgical technique
Early in the experience, a number of cases were performed via a right parasternal approach involving resection of the 2nd and 3rd costal cartilage (3). The majority of cases have since been performed with an upper hemisternotomy. Briefly, a 7-10 cm skin incision is made with the upper sternum divided and bone incision carried into the right 4th interspace or, occasionally, to the 3rd interspace. In a minority of cases, a “T” incision extending to the left interspace was made in order to facilitate exposure for concomitant mitral valve surgery. Alternatively, a lower hemisternotomy was performed due to a particularly low position of the aorta within the chest. Femoral arterial cannulation was used routinely in the early experience, but is now rarely employed for upper hemisternotomy. Axillary cannulation using a Dacron side graft is used selectively for those patients requiring arch reconstruction or in the presence of severe aortic calcification. Upper hemisternotomy also facilitates placement of the venous cannula via the chest incision. Venous cannulation is usually accomplished directly via the right atrium, or through the superior vena cava with a 3-stage cannula. For right anterior thoracotomy cases, venous cannulation is peripheral via the femoral vein, and for a few patients this also may be used for the upper hemisternotomy incision. Femoral artery cannulation has been used in a minority of cases, with the current preference being for direct aortic cannulation even with the right anterior thoracotomy approach, based on the favorable experience of a number of authors, including Glauber (8).
Myocardial protection was most often achieved with both antegrade and retrograde modified Buckberg cardioplegia, with the retrograde cannula placed through the incision without echocardiographic guidance. More recently, single dose Del Nido cardioplegia has been employed for most isolated valve cases, obviating the need for placement of the retrograde cannula into the coronary sinus. Conduct of the operation for a hemisternotomy case is similar to that during a full sternotomy aortic valve surgery, using standard instruments and procedures. Longer handled endoscopic instruments are utilized for mini-thoracotomy cases, and the Cor-Knot device (LSISolutions, Victor, NY) is commonly used for suture placement for AVR.
Statistics and follow-up
Data were retrieved from the prospective CVIR and from patients’ medical records, supplemented with information from the Echocardiography database. These data were approved for use in research by the institutional review board, with patient consent waived. All previous Cleveland Clinic studies involving aortic valve surgery and/or minimally invasive valve surgery were reviewed. Trends in the utilization of minimally invasive approaches, concomitant procedures performed, and outcomes for aortic valve surgery are presented as simple trends. Propensity matched outcomes for minimally invasive vs. standard aortic valve surgery for subsets of patients are presented as previously described (9).
Results
Utilization of minimally invasive approaches
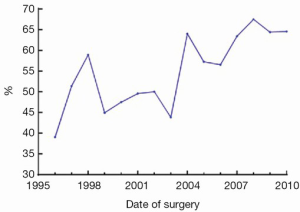
MIAVS was introduced to the Cleveland Clinic in 1996 by Cosgrove. In that year, these operations comprised 89 of a total of 718 aortic valve operations (12.4%), of which 66 (74.2%) were isolated aortic valves. The incision of choice for these early procedures was a right parasternal approach with peripheral cannulation (1). Despite a high rate of technical success, this approach was unsatisfactory secondary to a number of lung herniations requiring reoperation, and a stroke rate of 3%, which was attributed by the authors to the use of peripheral cannulation in atherosclerotic aortas (3). Over the next few years, surgeon preference combined with an evaluation of early and late outcome data evolved toward an upper hemisternotomy approach. With this, there was an increasing adoption of MIAVS as the preferred approach for isolated valve operations, and the advent of transcatheter valve replacement has not impacted the valve surgery volume (Table 1). In recent years, the number of combined operations has increased significantly, reflecting the increasing adoption of concomitant aortic procedures in particular.

Full table
Overall, the trend for both isolated aortic and mitral valve operations has been moving away from sternotomy and more toward less invasive approaches, with sternotomy now the incision of choice in the minority of isolated valve cases (Figure 2). The two greatest growth areas for the valve practice at present are primary and reoperative isolated aortic valves (data not shown). Since 2011, right anterior thoracotomy approaches have been used for a selected group of patients with aortic valve disease. To date, 48 of these operations have been performed with no hospital or 30-day mortality. Surgeon preference has been for routine preoperative three-dimensional (3D) imaging in these patients, and use of direct aortic cannulation where possible. Recently, this approach has also been used for sutureless valve replacement.
Concomitant procedures and standardized approach
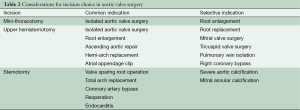
Operations performed in the early cohort of patients undergoing MIAVS included bioprosthetic AVRs, homograft aortic root replacements, and aortic valve repairs (3). With increasing experience, a large number of concomitant operations have been performed via MIAVS approaches. Currently, ascending aortoplasties, aortic root enlargements, aortic root replacements, ascending aortic replacements (with a cross-clamp) and hemiarch replacements (with circulatory arrest) are routinely performed in addition to aortic valve surgery via the upper hemisternotomy. AVR and root enlargement are performed via right anterior thoracotomy. To date, ascending aortic procedures, except for aortic endarterectomy for calcium, have not been performed via the anterior thoracotomy approach. While choice of incision is still dependent on surgeon preference, rough guidelines have evolved over time to govern incision selection (Table 2).
Full table
Mortality and MIAVS
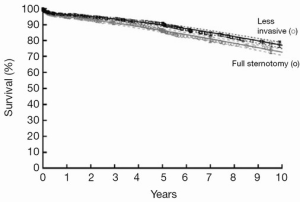
Cosgrove and Sabik reported no mortality in the first small series of parasternal aortic valve surgery, setting the standard that minimally invasive approaches should maintain the same safety profile as conventional operation (3). Over the period of the study, operative mortality for isolated aortic valve surgery has remained low, and declined gradually to 0.5% in 2013. Routine surveillance of aortic valve outcomes has been part of the practice at the Cleveland Clinic and continues to inform procedure and incision selection in these patients. In isolated aortic valve patients, overall mortality is higher for sternotomy than for MIAVS, reflecting patient selection. In a subset of propensity matched patients, early mortality was low (0.7%) and equivalent for both groups, and long term survival was identical (Figure 3) (4).
Benefits of MIAVS
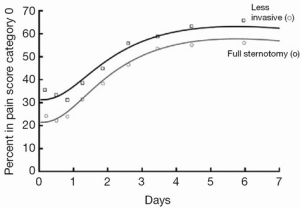
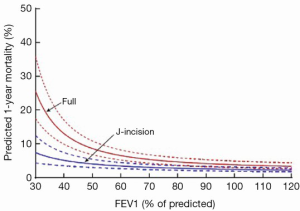
MIAVS patients report less pain after surgery, an effect that is sustained, though not dramatic (Figure 4). In addition, there is less utilization of narcotic pain medication in the first 2-3 days following surgery (4). MIAVS patients receive fewer blood and blood product transfusions, and are discharged from the hospital earlier than those with sternotomy (4). Of note, there is also a significant benefit in terms of pulmonary function as measured by bedside spirometry in the first 24-48 hours after surgery in MIAVS patients. This benefit in terms of early lung function may be one reason why patients with worse preoperative pulmonary function gain the most from MIAVS in comparison to sternotomy (Figure 5) (6).
Pitfalls of MIAVS
Complications related to the minimally invasive approach are related to the particularities of the incision itself, the lack of visualization of the entire heart and mediastinum and the increased challenge of navigating with decreased visibility. Lung herniation occurred in a subset of patients with parasternal approaches, requiring reoperation. Of the upper hemisternotomy patients, overall complication rates were not different compared with full sternotomy; however, in an analysis of 1,193 patients treated between 1995 and 2004, 34 (2.8%) patients underwent conversion to full sternotomy (4). Reasons for conversion included inadequate visualization (preclamp) and bleeding (postclamp). Of the patients with bleeding requiring conversion, the majority were related to coronary sinus injuries from placement of the retrograde cardioplegia cannula. An additional pitfall, which is not immediately apparent from the raw data, involves the frequency with which staff or trainee performs the procedure. Cross clamp times were shorter for upper hemisternotomy operations compared to full sternotomy for equivalent procedures, likely reflecting a tendency for the full sternotomy cases to be “teaching cases”.
Discussion
MIAVS has evolved over an 18-year experience at the Cleveland Clinic in terms of the preferred incision, cannulation strategy and method of myocardial protection. A significant driver of this gradual evolution has been the overarching concern that safety be maintained with the introduction of any new surgical technique. It is notable that the initial series of parasternal aortic valve procedures reported by Cosgrove and Sabik in 1996 were performed with zero mortality. Mortality in the current era for isolated AVR continues to be low and well under the expected mortality for similar patients in the Society for Thoracic Surgery (STS) database. It is in light of this difference that Cleveland Clinic surgeons have been slow to adopt right thoracotomy approaches for aortic valve surgery despite a large experience at some other institutions. Until recently, most large series of anterior thoracotomy AVRs have reported mortality in the range of 2-3%, which is in line with STS averages but higher than would be expected for large aortic valve centers. Glauber and colleagues have shown over several recent publications a declining mortality for the thoracotomy approach, which likely reflects increasing experience and the use of central aortic cannulation (8,10). Our small evolving experience with this approach suggests that it is a valuable tool in the MIAVS armamentarium in selected patients without need for a concomitant aortic procedure and with favorable anatomy on preoperative imaging. Our own data would suggest that upper hemisternotomy is a safe, reproducible operation that has demonstrable benefits in terms of earlier discharge, less blood utilization, decreased pain, and improved pulmonary outcomes. It may be considered the standard of care for isolated aortic valve operations and a reasonable option for concomitant procedures on the ascending aorta and root. Routine use of MIAVS combined with early conversion when necessary, and careful consideration of patients with potential contraindications results in excellent early and late outcomes that are at least comparable to sternotomy and possibly better.
The fact that MIAVS is safe and beneficial is certainly not as controversial as it once was, with a number of authors reporting large series with excellent mortality outcomes (10-13). In contrast to many other centers, we do not select this approach routinely for reoperations (14). In this cohort of patients in particular, we believe the reduction of operative risk and complications to be paramount. Safe sternal re-entry, adequate exposure, identification and isolation of patent internal thoracic artery grafts and meticulous myocardial protection are considered hallmarks of the Cleveland Clinic technique for cardiac reoperations (15). With this approach, the morbidity and mortality of reoperation approaches that of primary operation (16). As increased flexibility is often needed to deal safely with the pitfalls of a reoperative field, sternotomy remains the preferred approach for these cases. For upper hemisternotomy MIAVS, we have not seen the need for routine percutaneous femoral venous cannulation (12), as exposure for central venous cannulation is almost always possible.
This review of the Cleveland Clinic experience in MIAVS leaves a number of unanswered questions. One significant potential benefit of MIAVS is improved patient perceptions of quality of life and post-hospital outcomes such as return to work and functional capacity. Longitudinal follow-up in these patients is limited to metrics obtained by in-patients who return to the Cleveland Clinic for evaluation and by our routine telephone screening. Anecdotal evidence would suggest that patients perceive their operation to be smaller and less impactful to their lives when performed minimally invasively. However, long-term satisfaction and patient reported outcome data are lacking in our institution. In the current era where MIAVS is preferred for most surgeons for isolated aortic valve cases, we do not have the data to suggest why sternotomy was chosen in that subset. At this point there is not sufficient evidence to explain the recent sharp increase in concomitant procedures.
In light of the overwhelming evidence that aortic valve disease remains undertreated even in patients with well established diagnoses by echocardiography, the ability to offer MIAVS is an important tool for surgeons and cardiologists to increase acceptance of aortic valve surgery (17). It is clear that patients do not want sternotomy when it can be avoided. Many will seek to delay surgery if sternotomy is necessary even if the benefits of surgery are clear. If a safe, reproducible minimally invasive operation can be provided, including concomitant procedures when necessary and reasonable, this artificial barrier to surgery can be reduced. The Cleveland Clinic experience represents vast numbers (over 3,000) of minimally invasive aortic valve surgeries over 18 years, with proven safety and demonstrable benefits. Surgical technique has evolved to standardize the upper hemisternotomy approach as the most flexible, allowing most cases to be conducted with central arterial and venous cannulation, and single dose cardioplegia, in addition to allowing for ascending aortic and root repair with minimal modification in technique. The standardized approach outlined here is generalizable to any experienced valve surgeon who wishes to bring the benefits of MIAVS to affected patients (2).
It is as yet unclear whether mini-thoracotomy MIAVS will bring additional benefits without additional morbidity. Small series from other institutions suggest a benefit over upper hemisternotomy, and this approach is attractive to many patients. We postulate that routine central cannulation will be essential to avoid an increase in stroke rate with these approaches, as may be reflected in the higher mortality of some series (18). Our early experience suggests that these operations can be performed with aortic cannulation and a reasonable learning curve. Future propensity matched analysis will need to determine the risk/benefit ratio of mini-thoracotomy MIAVS compared with upper hemisternotomy. In addition, short and long-term patient reported quality of life data are still lacking. Several software and mobile tools are in development at the Cleveland Clinic that may allow surgeons to better track patients’ progress in terms of pain, return to work, activity level, and satisfaction once they leave the hospital.
Acknowledgements
Disclosure: Dr. Johnston receives honoraria from Edwards Lifesciences and St. Jude, and Dr. Roselli receives honoraria from Edwards Lifesciences, St Jude, Medtronic, and Sorin.
References
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014;147:6-14. [PubMed]
- Cosgrove DM 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998;65:1535-8; discussion 1538-9. [PubMed]
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012;144:852-8.e3.
- Atik FA, Svensson LG, Blackstone EH, et al. Less invasive versus conventional double-valve surgery: a propensity-matched comparison. J Thorac Cardiovasc Surg 2011;141:1461-8.e4.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2014;147:355-61.e5.
- Loor G, Desai MY, Roselli EE. Pre-operative 3D CT imaging for virtual planning of minimally invasive aortic valve surgery. JACC Cardiovasc Imaging 2013;6:269-71. [PubMed]
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6. [PubMed]
- Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg 2002;123:8-15. [PubMed]
- Glauber M, Miceli A, Bevilacqua S, et al. Minimally invasive aortic valve replacement via right anterior minithoracotomy: early outcomes and midterm follow-up. J Thorac Cardiovasc Surg 2011;142:1577-9. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. J Heart Valve Dis 2004;13:887-93. [PubMed]
- Tabata M, Umakanthan R, Cohn LH, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg 2008;33:537-41. [PubMed]
- Brinkman WT, Hoffman W, Dewey TM, et al. Aortic valve replacement surgery: comparison of outcomes in matched sternotomy and PORT ACCESS groups. Ann Thorac Surg 2010;90:131-5. [PubMed]
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [PubMed]
- Roselli EE, Pettersson GB, Blackstone EH, et al. Adverse events during reoperative cardiac surgery: frequency, characterization, and rescue. J Thorac Cardiovasc Surg 2008;135:316-23, 323.e1-6.
- Sabik JF 3rd, Blackstone EH, Houghtaling PL, et al. Is reoperation still a risk factor in coronary artery bypass surgery? Ann Thorac Surg 2005;80:1719-27. [PubMed]
- Kapadia SR, Goel SS, Svensson L, et al. Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg 2009;137:1430-5. [PubMed]
- Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 2011;91:79-84. [PubMed]