Combined open and endovascular treatment of thoracoabdominal aortic pathologies: a systematic review and meta-analysis
Background: A combined open-endovascular technique has emerged as an alternative treatment option
for thoracoabdominal pathologies. However, reported experiences from various medical centers have
been contradictory and heterogeneous. The aim of this study is to assess the mortality rate and various
complication rates associated with this approach.
Methods: An electronic health database search was performed on all articles published up to March of 2012
describing combined open-endovascular repair of thoracoabdominal pathologies. Studies were included in
the meta-analysis if they had ≥10 patients and reported the basic outcome criteria. End points of the metaanalysis
were defined as primary technical success, endoprosthesis related complications, 30-day/in-hospital
mortality, symptoms of spinal cord ischemia (SCI) and irreversible paraplegia, permanent renal function
impairment, and other major complications.
Results: Fourteen studies were deemed eligible for this meta-analysis with a total of 528 patients (68.0%
male, mean age 70.5 years). The mean follow-up period was 34.2 months. The pooled estimate for primary
technical success and visceral graft patency was 95.4% and 96.5% respectively. An endoleak developed in
106 (21.1%) patients in whom both stages had been completed. The pooled rate for symptomatic SCI was
7.0% and for irreversible paraplegia 4.4%. The pooled proportion for permanent renal failure was 7.0% and
for mesenteric ischemia 4.5%. Prolonged respiratory support and cardiac complications were observed in
a pooled rate of 7.8% and 4.6% respectively. The meta-analysis for 30-day/in-hospital mortality revealed a
pooled rate of 14.3%.
Conclusions: Although the hybrid technique for thoracoabdominal aortic pathology provides a less
invasive approach, the technique is still associated with a considerable morbidity and mortality rates. High
risk patients unfit to withstand open repair, are equally likely to suffer significant complications with the
hybrid procedure. The choice of the optimal treatment strategy for thoracoabdominal pathologies should be
carefully made on a patient to patient basis, assessing the clinical fitness and the anatomical suitability of each
patient. The hybrid approach should be reserved for high volume centers with accumulated experience and
high standards of perioperative management.
Key words: Aneurysm; thoracoabdominal aneurysm; visceral debranching
Introduction
Thoracoabdominal aortic aneurysms (TAAAs) typically present in elderly patients with serious comorbidities and their treatment remains a challenge. Because fewer than 40% of patients with large untreated TAAAs survive beyond three years, an operative approach is indicated with an encouraging life expectancy (1,2). The traditional repair of TAAAs with thoraco-laparotomy requires aortic cross clamping and is associated with considerable morbidity and mortality rates despite the significant advances in perioperative critical care and anesthetic and surgical techniques (3-6). In the endovascular area, further modalities for the management of TAAAs, including fenestrated or branched endografts, have been successfully applied (7,8). The intermediate-term patency and survival rates with this technique seem hopeful; however, morbidity and mortality is still considerable. In addition, the morphological parameters of the aneurysm or the urgent setting in cases of ruptured TAAAs can limit the endoluminal strategy application, because inadequate time is available to wait for a custom-made endoprosthesis.
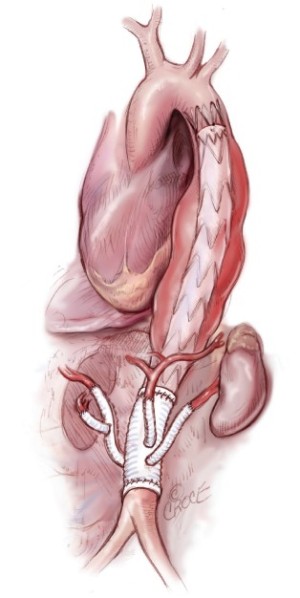
The hybrid repair of TAAAs has emerged as an alternative to traditional open repair (Figure 1). The hybrid procedure, first described in 1999 by Quiñones-Baldrich and colleagues, consists of debranching of the renal and visceral arteries followed by endovascular exclusion of the aneurysm (9). The technique was introduced with the intention of being reserved for a cohort of patients with compromised cardiac and respiratory reserves deemed unfit for open repair. The potential advantages of the hybrid approach include the avoidance of a thoracotomy and aortic cross-clamping. Many authors using the hybrid debranching strategy for the treatment of thoracoabdominal pathologies have reported discouraging results, and the initial enthusiasm for the technique has been replaced by doubt and uncertainty. A previous reported meta-analysis showed a 12.8% 30-day/in-hospital mortality rate, a 7.5% overall spinal cord ischemia (SCI) rate, and an 8.8% renal failure rate (10). Interestingly, a marked heterogeneity existed between different medical centers, with some of them demonstrating very low mortality and morbidity rates.
In the present study, a meta-analysis was conducted to investigate the technical success of the hybrid procedure as well as to explore the safety and efficacy of the technique in patients with TAAAs or other aortic diseases.
Materials and methods
Study design - search strategy
The present meta-analysis was conducted in accordance with the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (11). An extensive electronic health database search was performed on all articles published up to March of 2012 describing hybrid open-endovascular repair (HOER). The search was performed using exploded MeSH (medical subject heading) terms (“thoracic”, “abdominal”, “thoracoabdominal”, “aortic aneurysm”, “endovascular”, “stent-graft”, “endograft”, “visceral bypass graft”, “visceral revascularization”, “visceral debranching”, “hybrid”). Publications were retrieved through electronic search engines (Medline, Embase, Scopus, Google Scholar, Ovid, and the Cochrane Library). In addition, the reference lists of all retrieved articles were examined for further relevant series.
Definitions, eligibility, and exclusion criteria
HOER should consist of two stages: the open visceral debranching followed by the endovascular exclusion of the thoracoabdominal aortic pathology. The procedure can be performed either in a single stage or using a staged approach. An eligible study for the present meta-analysis should: (I) involve visceral bypass followed by aortic stent-graft implantation, (II) provide baseline characteristics of the recruited patients, (III) state the incidence of at least one of the basic outcome criteria, and (IV) report on a series of ≥10 patients. Exclusion criteria were the following: articles in languages other than English, reports on HOER with aortic arch debranching involvement, case reports, and a series of <10 patients. When multiple publications on the same patient sample were identified or study populations overlapped, only the latest report was included unless the reported outcomes were mutually exclusive. Furthermore, in several studies, patients with combined visceral debranching and endovascular exclusion of thoracoabdominal pathologies were analyzed as a subgroup of a wider patient sample. These studies were excluded from the present meta-analysis because data regarding this subgroup of patients were not separately provided. All studies were assessed by two reviewers (K.M. and S.M.). The available data were extracted and analyzed, and a consensus was reached if discrepancies were observed.
Data extraction
The eligible studies for this meta-analysis were independently assessed by two reviewers (K.M. and S.M.) for the study design: first author, year of publication, type of study; patient sample: patient gender, mean age, comorbidities, indication for HOER, type of TAAA [according to the Crawford classification, modified by Safi (12)], maximal diameter of the aneurysm, stages of the procedure (and the intraprocedural interval if a staged approach was applied). Study end points assessed included primary technical success, mean length of intensive care unit (ICU) and hospital stay, mean follow-up period, endoprosthesis related complications, 30-day/in-hospital mortality, symptomatic SCI and permanent paraplegia, permanent renal function impairment (≥25% rise in serum creatinine or need for dialysis), mesenteric ischemia, prolonged respiratory support (>5 days), and cardiac complications. If discrepant results were obtained, the articles were re-analyzed by the two reviewers and a consensus was reached.
Statistical analyses
Standard descriptive statistics (reported as means with 95% confidence intervals) were used to summarize demographical and baseline data of the recruited patients from all eligible studies. Separate meta-analysis was carried out on all included studies for technical success, SCI symptoms, renal insufficiency, need for prolonged respiratory support, and 30-day/in-hospital mortality. The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions using the random effects model proposed by DerSimonian-Laird (13). Heterogeneity among studies was estimated using the chi-square test and Cochran Q score (reported as I2 and representing the percent value of the heterogeneity). Funnel plots were constructed, and the identified extreme studies were excluded to increase the robustness of our analyses. Frequency study-specific estimates were pooled and are reported as proportions with 95% confidence intervals (95% CI). The possibility of publication bias was assessed for both aims using the Begg-Mazumdar adjusted rank correlation test (14). The meta-analyses and the publication bias assessment were conducted using the StatsDirect statistical software (StatsDirect Ltd, UK).
Results
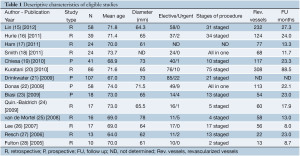
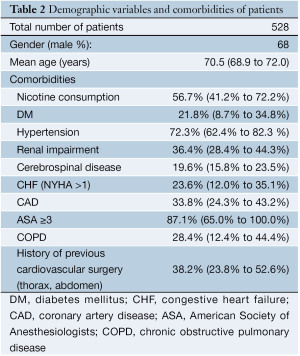
After a review of the abstracts, 157 out of 465 total articles of interest were eventually deemed relevant. Out of these 157 articles, 143 publications were excluded in the subsequent evaluation and the inclusion criteria application (Figure 2). Fourteen studies were deemed eligible for this meta-analysis, including 10 retrospective and four prospective (Table 1) (15-28). A total of 528 patients (68.0% male, mean age 70.5 years, 95% CI: 68.9-72.0 years) were analyzed. Demographic variables and comorbidities of the patients are detailed in Table 2. The majority of the patients (64.3%) underwent HOER attributable to a degenerative thoracoabdominal aneurysm; 18.2% attributable to aortic dissection; 5.9% attributable to visceral aortic patch aneurysm after open repair; 3.6% attributable to secondary aneurysms associated with connective tissue disorders (Marfan syndrome or Ehlers-Danlos syndrome); 0.9% attributable to mycotic aneurysms; and 7.0% attributable to other aortic pathologies (i.e., penetrating ulcers, intramural hematomas).
Full table
Full table
The extent of the TAAA was determined in 397/528 (75.2%) patients. In particular, 12.8% of the TAAAs were of type I, 23.2% of type II, 38.0% of type III, 23.7% of type IV, and 11.1% of type V. The mean aneurysm diameter was 68.0 mm (95% CI: 65.2-70.9 mm). Almost 89% of the patients were referred for elective treatment, while 11.2% of them experienced symptomatic or ruptured TAAA before admission. A single-stage approach was followed in 47.5% of the patients whereas 52.5% underwent a staged procedure with a mean intraprocedural interval of 29.6 days (95% CI: 4.2-54.9 days). Mean ICU stay was 6.2 days (95% CI: 4.7-7.6 days), mean hospital stay was 20.8 days (95% CI: 15.8-25.8 days), and mean follow-up period was 34.2 months (95% CI: 16.6- 51.8 months).
Primary technical success
With respect to the primary technical success, defined as completed visceral debranching and successful stentgraft deployment, the pooled estimate was 95.4% (95% CI: 91.8% to 98.0%) (Figure 3). In three cases, the operation was not completed because of perioperative instability, whereas in one case the debranching procedure was abandoned because of a restricted flow rate that was considered insufficient for safe revascularization of the renal and visceral arteries. One patient did not undergo aortic endograft placement because of complications from the debranching procedure. Twelve patients died due to procedure-related complications after the first stage. Six more patients with successful visceral rerouting died from aortic rupture while waiting the endovascular stage, whereas four patients refused it.
Out of the 502 patients for whom both stages of the procedure were completed, 106 (21.1%) experienced an endoleak during the mean follow-up of 34.2 months (95% CI: 16.6 to 51.8 months). In particular, 111 endoleaks were depicted in follow-up CT scans: 32 type I, 66 type II, and 13 type III. Reintervention was required in 26 of them.
Visceral graft patency
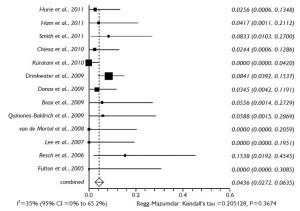
According to the aneurysm extent and the sufficiency of the collateral supply through the superior mesenteric artery (SMA), the celiac trunk (CT) was selectively revasularized. The inflow sites were retrograde, i.e. native iliac arteries, distal aorta, or infrarenal prosthetic graft in patients with prior aortic surgery, and antegrade, i.e. ascending or supraceliac aorta. The utilized grafts were autologous vein grafts, synthetic (polyester or ePTFE), or Viabahn covered stent-grafts (Gore & Associates, Flagstaff, AZ). A total of 1,302 visceral grafts to visceral or renal arteries (RAs) were identified: 286 to CT, 347 to SMA, and 669 to RAs. The pooled rate for visceral graft patency during the mean follow-up of 34.2 months (95% CI: 16.6-51.8 months) was 96.5% (95% CI: 94.5-98.0%) (Figure 4). Forty-one grafts were occluded: 4 to CT, 12 to SMA, and 25 to RAs.
Morbidity and mortality rates
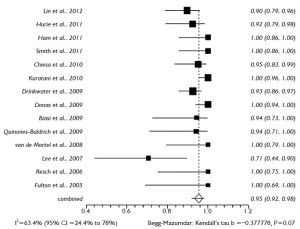
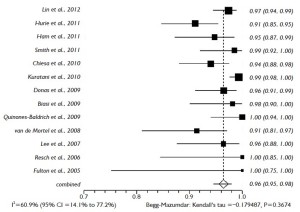
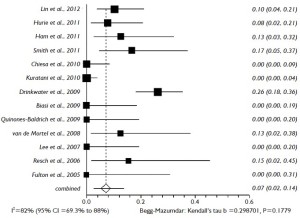
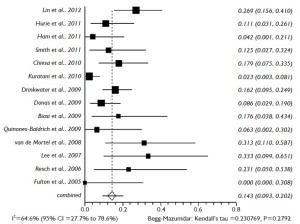
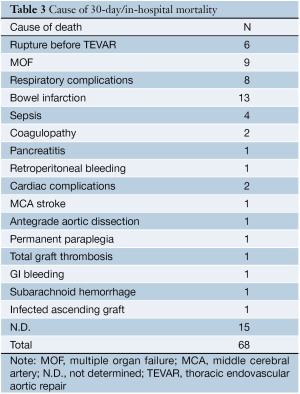
The proportional meta-analysis showed a pooled rate for symptomatic SCI of 7.0% (95% CI: 4.9-9.5%), and in 4.4% (95% CI: 2.7-6.3%), the SCI symptoms were irreversible (Figure 5). The pooled estimate for permanent renal failure requiring dialysis was 7.0% (95% CI: 2.4-13.8%) (Figure 6). The pooled estimate for mesenteric ischemia was 4.5% (95% CI: 2.3-7.3%), whereas the pooled rate for prolonged respiratory support (>5 days) was 7.8% (95% CI: 3.3-14.2%). Cardiac complications were present in a pooled rate of 4.6% (95% CI: 2.0-8.3%). The meta-analysis for 30- day/in-hospital mortality revealed a pooled rate of 14.3% (95% CI: 9.3-20.2%) (Figure 7). A total of 68 patients died during the early postoperative period. The causes of death are detailed in Table 3.
Full table
Discussion
Theoretically, the hybrid repair for thoracoabdominal aortic pathology offers a less morbid technique as it avoids a thoracotomy, cross clamping of the aorta, singlelung ventilation, and prolonged end-organ ischemia. This anticipation was not confirmed in clinical practice, as practitioners of the technique reported considerable mortality and complication rates. Undoubtedly, the extensive dissection required for this procedure and the surgical maneuvers for the preparation of the aorta result in significant organ and tissue trauma.
A previous meta-analysis on hybrid repair of thoracoabdominal pathologies showed differing results, raising a debate for the role of the technique (10). This meta-analysis was performed by attempting to investigate further concerns and aspects that may influence the safety of the technique. Intending to avert bias of the small case series, the present analysis excluded case reports or small case series. Consistent with the previous reported metaanalysis, significant heterogeneity was found among studies on morbidity and mortality rates. This updated meta-analysis revealed a pooled rate for 30-day mortality of 14.3%. Irreversible SCI occurred in 4.4% of patients, whereas the rate for permanent renal failure requiring dialysis was 7.0%. Bowel ischemia and cardiopulmonary complications also occurred at a considerable rate. Recently presented data in the Society for Vascular Surgery (SVS) meeting 2012 from the North American Complex Abdominal Aortic Debranching (NACAAD) Registry showed similar results. The 30-day mortality was 16% among patients treated for TAAAs. Spinal cord ischemia occurred in 14% of patients and was associated with the extent of aortic disease and aneurysm rupture (29).
Interpretation of these results must take in to account several parameters. Thirteen years after its introduction, the technique continues to be used with ambiguous results. A reason for the substantial mortality associated with the debranching technique is that the majority of these patients are elderly, seriously ill, and with major comorbidities that cannot withstand the hybrid reconstruction. This analysis had a mean age of treated patients of 70.5 years while more than 85% had an American Society of Anesthesiologists (ASA) physical status ≥3. Approximately 10% of patients in this analysis underwent urgent operations for a symptomatic or ruptured aneurysm. A third interesting point is that the debranching hybrid technique has been adopted by centers with little previous experience in treating patients with TAAAs. A learning curve and an organized perioperative background incorporating several adjunctive and protective measures for best management are required. Consistent evidence exists in the literature showing better results from a handful of higher volume institutions in both the U.S. and Europe.
Hybrid repair can be applied using a single-stage (during the same anesthesia admission) procedure or a two-stage approach. In our review study, a single-stage strategy was followed in 47.5% of the patients whereas 52.5% underwent a staged procedure with a mean intraprocedural interval of 29.6 days. Devotees of the single-stage approach support the concept that such procedures eliminate the risk of rupture between stages and the potential patient’s consent withdrawal for the endovascular stage (21). Extracted relative data from our meta-analysis revealed that six patients out of the 273 scheduled to undergo a staged procedure succumbed because of aneurysm rupture while waiting for the second stage of the procedure, whereas four other patients refused the procedure. In contrast, a single-stage strategy is described as being associated with longer cumulative ICU and hospital stay and with higher morbidity rate (15). Therefore, a single-stage procedure should be considered for patients with excessively large aneurysms at risk for rupture whereas a staged approach seems more reasonable for high-risk patients who require extensive aortic reconstruction.
A further concern is the durability and long-term outcome of the technique which is related either to endograft complications or to visceral graft occlusion. Our meta-analysis revealed a visceral graft patency exceeding 96% during the mean follow-up of 34.2 months, whereas 21.1% (106/502) of the patients had an endoleak. In 5% of patients with endoleak, a reintervention was required. In the study with the longest follow-up period (88.5 months, n=86) by Kuratani et al., two procedure-related late deaths were recorded; one patient expired because of a visceral graft occlusion and resultant mesenteric necrosis, whereas the other presented with a fatal graft infection (20) In the study by Lin et al. (15) there was no aneurysm-related death in a mean follow up of 27.3 months, whereas Hurie et al. (16) reported a late aneurysm related mortality rate of 12.8%.
Conclusions
Despite providing a less invasive approach to treat thoracoabdominal aortic pathology, the hybrid technique is still associated with a considerable morbidity and mortality rate. Despite the improvements in totally endovascular techniques for the treatment of thoracoabdominal pathologies, hybrid procedures may continue have a role in those patients anatomically unsuitable for fenestrated and side branched endografts. The technique should be reserved for high volume centers with accumulated experience and high standards of perioperative management.
Acknowledgements
The authors thank Ms. Beth Croce, ACS Illustration Editor and Dr. Ashutosh Hardikar, ACS Art of Operative Techniques Section Editor, for making the professional medical illustration.
Disclosure: The authors declare no conflict of interest.
References
- Schermerhorn ML, Giles KA, Hamdan AD, et al. Mesenteric revascularization: management and outcomes in the United States, 1988-2006. J Vasc Surg 2009;50:341-8.
- Cherr GS, Hansen KJ, Craven TE, et al. Surgical management of atherosclerotic renovascular disease. J Vasc Surg 2002;35:236-45.
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4;discussion S890-2.
- Rigberg DA, McGory ML, Zingmond DS, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg 2006;43:217-22; discussion 223.
- Schepens MA, Kelder JC, Morshuis WJ, et al. Long-term follow-up after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2007;83:S851-5; discussion S890-2.
- Etz CD, Luehr M, Kari FA, et al. Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: does critical spinal cord ischemia occur postoperatively? J Thorac Cardiovasc Surg 2008;135:324-30.
- Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 2010;140:S171-8.
- Haulon S, D’Elia P, O’Brien N, et al. Endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2010;39:171-8.
- Quiñones-Baldrich WJ, Panetta TF, Vescera CL, et al. Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg 1999;30:555-60.
- Moulakakis KG, Mylonas SN, Avgerinos ED, et al. Hybrid open endovascular technique for aortic thoracoabdominal pathologies. Circulation 2011;124:2670-80.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12.
- Safi HJ. How I do it: thoracoabdominal aortic aneurysm graft replacement. Cardiovasc Surg 1999;7:607-13.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101.
- Lin PH, Kougias P, Bechara CF, et al. Clinical outcome of staged versus combined treatment approach of hybrid repair of thoracoabdominal aortic aneurysm with visceral vessel debranching and aortic endograft exclusion. Perspect Vasc Surg Endovasc Ther 2012;24:5-13.
- Hurie J, Patel HJ, Criado E, et al. Postoperative fluid collection after hybrid debranching and endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2011;54:1623-8.
- Ham SW, Chong T, Moos J, et al. Arch and visceral/ renal debranching combined with endovascular repair for thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg 2011;54:30-40; discussion 40-1.
- Smith TA, Gatens S, Andres M, et al. Hybrid repair of thoracoabdominal aortic aneurysms involving the visceral vessels: comparative analysis between number of vessels reconstructed, conduit, and gender. Ann Vasc Surg 2011;25:64-70.
- Chiesa R, Tshomba Y, Marone EM, et al. Hybrid procedures for the treatment of thoracoabdominal aortic aneurysms and dissections. J Cardiovasc Surg (Torino) 2010;51:821-32.
- Kuratani T, Kato M, Shirakawa Y, et al. Long-term results of hybrid endovascular repair for thoraco-abdominal aortic aneurysms. Eur J Cardiothorac Surg 2010;38:299-304.
- Drinkwater SL, Böckler D, Eckstein H, et al. The visceral hybrid repair of thoraco-abdominal aortic aneurysms- -a collaborative approach. Eur J Vasc Endovasc Surg 2009;38:578-85.
- Donas KP, Lachat M, Rancic Z, et al. Early and midterm outcome of a novel technique to simplify the hybrid procedures in the treatment of thoracoabdominal and pararenal aortic aneurysms. J Vasc Surg 2009;50:1280-4.
- Biasi L, Ali T, Loosemore T, et al. Hybrid repair of complex thoracoabdominal aortic aneurysms using applied endovascular strategies combined with visceral and renal revascularization. J Thorac Cardiovasc Surg 2009;138:1331-8.
- Quinones-Baldrich W, Jimenez JC, DeRubertis B, et al. Combined endovascular and surgical approach (CESA) to thoracoabdominal aortic pathology: A 10-year experience. J Vasc Surg 2009;49:1125-34.
- van de Mortel RH, Vahl AC, Balm R, et al. Collective experience with hybrid procedures for suprarenal and thoracoabdominal aneurysms. Vascular 2008;16:140-6.
- Lee WA, Brown MP, Martin TD, et al. Early results after staged repair of thoracoabdominal aortic aneurysms. J Am Coll Surg 2007;205:420-31.
- Resch TA, Greenberg RK, Lyden SP, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther 2006;13:481-9.
- Fulton JJ, Farber MA, Marston WA, et al. Endovascular stent-graft repair of pararenal and type IV thoracoabdominal aortic aneurysms with adjunctive visceral reconstruction. J Vasc Surg 2005;41:191-8.
- Oderich GS, Timaran C, Farber M, et al. RR14. Spinal Cord Injury After Hybrid Endovascular Repair of Thoracoabdominal Aortic Aneurysms in the North American Complex Abdominal Aortic Debranching (NACAAD) Registry. 2012Vascular Annual Meeting. June 7- 9, 2012 , Washington, D.C.,USA