Interventions on the aortic valve and proximal thoracic aorta through a minimally invasive approach
Clinical vignette
A 64-year-old active male who regularly runs noted that he was developing exercise intolerance with increasing dyspnea on inclines. He presented to his primary care physician for a health maintenance visit and a murmur was detected. The rest of his physical examination and lab work-up was unremarkable. Echocardiography demonstrated mild (1+) aortic insufficiency and moderate aortic stenosis (mean gradient of 22 mmHg) in the setting of a bicuspid aortic valve of the most common type with fusion of the right and left cusps, two well-formed commissures, and a single raphe. Additional cross-sectional imaging with contrast enhanced, ECG-gated computed tomography demonstrated a 5.4 cm ascending aneurysm with an ascending phenotype (i.e., preserved root architecture and sinutubular junction and normal arch at the level of the left carotid artery) with some calcium on the cusps of the valve. He was referred for surgical repair. Cardiac catheterization revealed minimal coronary artery disease and he was recommended to undergo minimally invasive aortic valve repair or replacement (dependent on direct inspection) with ascending and hemiarch aneurysm repair.
Surgical techniques
Patient selection
For patients with aortic valve disease in combination with an ascending aneurysm, we frequently recommend a minimally invasive repair (1). For patients with isolated aortic valve disease requiring only replacement or repair, we increasingly use a mini right thoracotomy approach. For patients with combined aortic disease, we prefer the mini sternotomy approach. This includes patients requiring valve replacement or repair in combination with ascending aortic aneurysm repair, aortic root replacement as a Bentall procedure, and proximal aortic arch surgery requiring circulatory arrest. Young patients with a bicuspid aortic valve and associated aortopathy are typically common candidates, but any pathology may be amenable including congenital aortic anomalies like Kommerel’s diverticulum requiring frozen elephant trunk repair (2). One exception is for patients requiring valve-sparing root replacement (modified David’s procedure), in which we perform the operation through a full sternotomy in order to ensure adequate exposure.
Preparation
Careful review of contrast enhanced three-dimensional computed tomography (3D CT) imaging is required by the operating surgeon to understand the details of the patient’s anatomy and the morphology of the aneurysm and the valve (3) (Figure 1). All patients undergo preoperative cardiac catheterization for the possibility of concomitant coronary revascularization, and echocardiography to assess valve function. Additionally, patients undergo pulmonary function testing, brain imaging, and routine lab work.
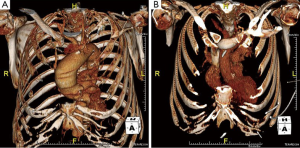
In patients with a bicuspid aortic valve, careful analysis of the valve and the pattern of aortopathy is critical to the decision-making process. A modified David’s reimplantation procedure is preferred instead of a minimally invasive aortic valve surgery (MIAVS) if the patient has a predominantly insufficient bicuspid aortic valve without calcification (i.e., a high likelihood of repair) and the root phenotype of aortopathy is annuloaortic ectasia (annulus >25 mm) or dilated sinuses (>40 mm). All other presentations of bicuspid valve disease with aortopathy are amenable to MIAVS (4).
Exposition
The midline is marked and bony landmarks identified including the sternal notch, angle of Louis and interspaces. The skin incision is marked to be 8 cm long and is centered over the space between the sternal notch and the fourth interspace (Video 1). After making the skin incision, finger retractors are used to manipulate the skin and subcutaneous tissue over the length of bone to be divided, and tissue is cleared off the sternal notch and the right edge of the sternum within the fourth interspace. The saw is maintained in the centerline until the level of the fourth rib at which point the cut is angled into the fourth interspace. Care is taken to avoid injury to the right internal thoracic artery. Careful hemostasis is maintained along the edges of the bone and the bone marrow. The thymus is divided between ties and the pericardium is opened from the brachiocephalic vein to the lowest point visible caudally. Three pericardial stay sutures are placed along each edge of the pericardium and individually elevated to bring the edge of pericardium over the cut surface of the sternum which is then carefully clipped to the drapes. Elevating the pericardium optimizes exposure of the aortic root, but care is taken to do this slowly and monitor hemodynamics as pulling excessively on the pericardium may reduce preload and cause hypotension. A mini Finochietto retractor is then placed to spread the sternum open.
To optimize distal ascending and proximal arch exposure, dissection is carried out along the underside of the brachiocephalic vein, taking down the pericardial reflections on the proximal arch and to the right of the brachiocephalic artery. In order to facilitate exposure of the proximal aortic arch, a self-retaining lift retractor (Hercules; Estech, West Chester, OH, USA) is fixed to the side of the mini-Finochietto retractor. The retractor arm is placed between the underside of the brachiocephalic vein and the aortic arch and then lifted cranially and secured.
Cannulation is performed in the distal ascending aorta within the aneurysm. This site is selected distal enough on the aorta to allow for placement of a cross clamp during cooling and proximal enough so as not to traumatize the aortic edge at the level of the distal anastomosis. Venous cannulation is performed with a two-stage cannula placed through the right atrial appendage into the inferior vena cava. Additionally, a right angle cannula is placed into the superior vena cava to facilitate drainage and the delivery of retrograde brain perfusion if preferred by the surgeon. Vacuum assisted drainage is utilized during cardiopulmonary bypass.
Operation
After safe cannulation, cardiopulmonary bypass is initiated and cooling is begun to less than 20 degrees Celsius. During the cooling phase, additional dissection along the proximal arch is performed and exposure of the aortic root is optimized. When the heart fibrillates, the aneurysm is clamped and a single shot of Del Nido cardioplegia is delivered antegrade for a total of about one liter of volume. If the patient has significant aortic insufficiency, then up to half of the dose is given into the aortic root to arrest or slow the heart, and the rest is given with direct injection into the coronary arteries after opening the aorta above the sinutubular junction. During the rest of the cooling phase, the aorta is transected at the level of the cross clamp and across the sinutubular junction. The valve is then carefully inspected.
Although this patient did not have severe dysfunction of his bicuspid aortic valve, there was calcific degeneration and fibrosis involving both the conjoined cusps and the non-coronary cusps suggesting that the durability of a repair would be limited. Given his age in the sixties, valve replacement with a bioprosthesis was chosen.
The size of the proximal site of graft anastomosis is assessed in order to inform selection of surgical graft (28 mm Gelweave, Terumo, Ann Arbor, MI, USA). The graft with a 10 mm side limb is selected and prepared by beveling it across the distal edge just beyond the side limb, and a 3/8th inch by 3/8th inch tubing connector is fixed into the side limb of the graft for later recannulation.
Once an adequate level of cooling of the patient is achieved [at least twenty minutes with electroencephalographic silence as assessed by bispectral index (BIS) monitor], the superior vena cava is clamped near its junction with the right atrium and the circulation is arrested. The cross clamp and the cannula are removed from the aorta and retrograde brain perfusion is run at a rate of approximately 150-300 mL/minute. The proximal arch is transected from the base of the brachiocephalic artery to the lesser curve and the distal aortic graft anastomosis is performed. The anastomosis is performed using a 5-0 polypropylene running suture supported with a strip of bovine pericardium. Typical circulatory arrest times range between 8 to 13 minutes. Once the anastomosis is completed, the 10 mm side limb is readily cannulated and the arch is de-aired. The graft is clamped more proximally, and full flow is then resumed. Re-warming is begun once any metabolic acidosis has been cleared.
Great care is taken to secure hemostasis along the distal aortic suture line during this phase of the operation, when it is best seen. If this is not optimized at this point, then it may be very difficult to do so through a small incision after the reconstruction is completed. The clamp is then repositioned closer to the suture line and attention is returned to the aortic root.
The valve is excised and the annulus debrided. The left ventricle and aortic root is gently irrigated with saline so as not to create too many bubbles. The aortic valve is replaced (25 mm Trifecta; St. Jude, St. Paul, MN, USA) using a running technique with three 3-0 polypropylene sutures on RB1 needles. After the valve is positioned, the sutures tightened and secured, the valve replacement is carefully inspected. The proximal graft anastomosis is then performed to the sinutubular junction again with running 5-0 polypropylene suture and a strip of bovine pericardium.
Typically at this stage an adequate level of re-warming of the patient has been achieved (35.5 degrees Celsius in the bladder), meaning that the patient can then be weaned from cardiopulmonary bypass. Transesophageal echocardiography is performed to assess valve integrity, ventricular function, and adequate de-airing. Final hemostasis along the suture lines is secured while heparin is reversed. Temporary ventricular pacemaker wires are placed and a mediastinal chest tube is inserted centrally at the site of the previous placed CO2 line. Chest closure is performed with 5 to 6 wires.
Comments
A minimally invasive approach to valve surgery was first described at the Cleveland Clinic in 1996. Since then, minimally invasive approaches to the aortic valve and proximal aorta have become a mainstay at our institution and others (1). For the last five years we have performed over 1,700 aortic valve operations per year and nearly one third of those have been performed using minimally invasive techniques. The trend of increased use of these approaches has occurred because the techniques are: reproducible, widely applicable to many patients and disease processes including those involving the proximal aorta, and associated with superior outcomes.
Clinical results
Of the 520 minimally invasive operations involving the aortic valve performed at our institution in 2013, 305 were multi-component procedures and many of them included an operation on the proximal aorta. The mortality for the last several years at our institution has been less than 0.5% for isolated aortic valve surgery including reoperations, and less than 1.5% for aortic valve surgery combined with other procedures including coronary bypass grafting. The mortality for 228 patients who underwent ascending aortic surgery in the setting of a bicuspid valve was 0.6% in 2013. Furthermore, the risk of stroke has been low at about 1.1% for aortic valve surgery and less than 3% for all patients undergoing aortic operations, and even less in those undergoing minimally invasive operations. A recently published propensity matched analysis demonstrated no difference in death, stroke, or renal failure when MIAVS was compared to full sternotomy, and instead it showed a significant decrease in complications and resource utilization (1).
Advantages
The minimally invasive approach described allows for excellent access to the entire proximal aorta and the aortic valve while maintaining the integrity of the diaphragmatic attachments to the chest wall. As such, the patients have less respiratory compromise and may experience less pain and bleeding, reducing recovery time. Additional advantages include improved aesthetics and easier access in the event that reoperation becomes necessary later. This approach does not significantly extend the time of the operation nor the period of circulatory arrest.
Caveats
Obtaining optimal hemostasis at the distal aortic suture line before proceeding with the proximal reconstruction is critical. It is during this point in the operation when that suture line is best seen. Although root replacement can easily be performed through this approach, the exposure may not be ideal when a more complex reconstruction like a David’s procedure is indicated. Using preoperative CT scan reconstructions to plan the exposure is important to ensure safe patient selection, as some patients with ascending aneurysms may have significant leftward and caudal shift in the lie of their heart and aorto-ventricular junction (3).
Acknowledgements
Dr. Roselli receives honoraria from Edwards Lifesciences, Medtronic, Sorin, and St Jude.
Disclosure: The author declares no conflict of interest.
References
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012;144:852-858.e3.
- Idrees J, Keshavamurthy S, Subramanian S, et al. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg 2014;147:973-6. [PubMed]
- Loor G, Desai MY, Roselli EE. Pre-operative 3D CT imaging for virtual planning of minimally invasive aortic valve surgery. JACC Cardiovasc Imaging 2013;6:269-71. [PubMed]
- Loor G, Roselli E. Mini-Sternotomy for Hemiarch and Bicuspid Aortic Valve (BAV) Repair. Available online: http://www.ctsnet.org/article/mini-sternotomy-hemiarch-and-bicuspid-aortic-valve-bav-repair





