Patient-prosthesis mismatch after transapical aortic valve implantation
Introduction
Patientprosthesis mismatch (PPM) was described by Rahimtoola in 1978 (1) as an effective prosthetic valve area that is less than that of a normal human valve. Despite technical efforts to optimize valve prostheses, their rheological properties are not comparable with those of native human valves and aortic stenosis will occur in a normally functioning prosthesis that is too small for the patient. PPM is associated with decreased regression of left ventricular hypertrophy, reduced coronary flow reserve, increased incidence of congestive heart failure, diminished functional capacity, and increased risk of early and late mortality (2,3).
This paper is a brief review of the clinical significance of PPM, with reference to transcatether aortic valve implantation (TAVI).
Determination of effective orifice area
A clinically used parameter for the detection and severity assessment of PPM is effective orifice area (EOA) indexed with body surface area (iEOA) (4). The continuity equation, based on the concept that the stroke volume in LVOT and across the aortic valve is equal, is used to determine EOA. Stroke volume in Doppler echocardiography is calculated as crosssectional area (CSA) multiplied by the average flow velocity during the ejection period (velocity time integral, VTI). This requires three echocardiographic measurements (Figure 1): (I) Diameter of left ventricular outflow tract (LVOT); (II) Velocity in LVOT using pulsed wave Doppler; (III) Velocity across the aortic valve using continuous wave Doppler.
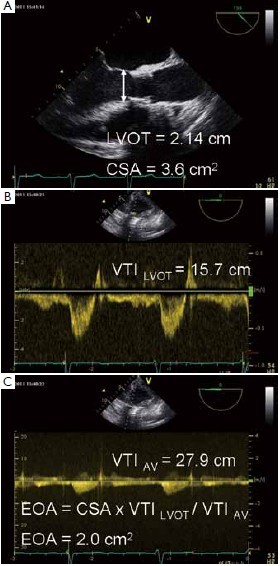
The resulting calculated values (VTILVOT - velocity time integral measured in LVOT, CSALVOT – cross sectional area of LVOT, and VTIAV – velocity time integral of velocity measured across the aortic valve) are included in the continuity equation to obtain the EOA: EOA = VTILVOT x CSALVOT / VTIAV.
Measurement of LVOT diameter depends on exact imaging (zoom mode) to avoid under- or overestimation. In TAVI patients, the consensus is that diameter has to be measured just proximal to the prosthetic stent (5-8), which is uniquely defined and reproducible.
EOA is usually assessed with transthoracic echocardiography preoperatively, but transesophageal echocardiography (TEE) can be used for immediate post-procedural quantification (7). Potential advantages of TEE are better anatomical imaging and data acquisition immediately after valve implantation. Geometrical orifice area (GOA) (measured as area tracing in the aortic short axis view), has been show to overestimate the “functional area” of valve prostheses. The mean EOA/ GOA ratio is 0.85±0.18 (7). However, GOA measurements may be useful to verify EOA calculation.
PPM classification
For PPM assessment, EOA is indexed with body surface area (iEOA in cm2/m2).
Normal iEOA values are >0.85 cm2/m2. Echocardiography guidelines (9) classified PPM as severe if iEOA 2/m2 and moderate if iEOA is from 0.65 to 0.85 cm2/m2.
PPM and outcome
After surgical aortic valve replacement (sAVR), the incidence of moderate PPM is 20-70% and severe PPM 2-11%. Severe PPM is related to adverse clinical outcomes (10). Blais (11) and Rabus (12) identified severe PPM as an independent predictor of short-term mortality following sAVR. Although there are no direct comparative trials, PPM may be less frequent after TAVI than after sAVR because of better hemodynamic performance of the bioprosthesis. After TAVI, moderate PPM has been reported in 23-30% of cases and severe PPM in 2-16% (5,7,8,13) .
For the CoreValve prosthesis (Medtronic, Minneapolis, MN) Jilaihavie (6) reported 30% moderate and 2% severe PPM in 50 patients, while Tzikas (8) reported moderate and severe PPM in 23% and 16% of 75 patients respectively. For the Edwards Sapien valves (Edwards Lifesciences, Irvine, CA), Clavel et al. (5) found severe PPM in 10% of 50 patients. This was consistent with our findings of moderate PPM in 27% and severe PPM in 7.6% of 278 patients (7).
While severe PPM significant affects 3-month mortality moderate PPM adversely affects LV mass regression, LV filling pressure, and clinical ourcomes (13). Previously, retrospective studies demonstrated inconsisitent findings on the impact of PPM on clinical outcomes. This was likely due to differences in the definitions of PPM used in these studies (EOA, GOA, in vitro orifice area). To date, there are relatively few studies of PPM following TAVI. Only one study (7), has demonstrated a significant impact of severe PPM on survival. Within the subgroup of patients with severe PPM, those with a combination of higher transvalvular gradient and LVEF less than 50% presented a strong trend toward decreased 3-month survival. However, after 3 months, the survival in patients with severe, moderate and no PPM was similar.
TAVIs are usually supported with peri-procedural echocardiography; providing an opportunity to measure iEOA, valve hemodynamics and quantification of systolic and diastolic ventricular function. These quantitative values can be used for post-procedural risk stratification.
TAVI-determining optimal prosthesis size
Pibarot (10) suggested an algorithm to predict iEOA related to expected EOA for the type of surgical prosthesis in patients scheduled for surgical aortic valve replacement (sAVR). When the predicted iEOA is below 0.65 cm2/m2, alternative options should be considered. A database of EOAs for every bioprosthesis for TAVI is available, but the actual EOA following TAVI depends on a number of factors, such as annulus size, degree of calcification, dilatation and implantation technique. Future databases may allow the estimation of annulus size and degree of calcification on iEOA and may provide algorithms for the prediction of post-procedural iEOA.
To avoid possible complications of TAVI (such as annulus rupture) (14) and the risk of post-procedural aortic regurgitation (15) it is essential to choose the optimal prosthesis size. Multi-modality imaging including intraprocedural transesophageal echocardiography facilitates these decisions. Interestingly, Kalavrouziotis et al. (16) reported that TAVI (Edwards Sapien valve) in patients with a small annulus (
In the future, another approach in patients with a small annulus and severe calcification could be open direct transaortic valve implantation. Using cardiopulmonary bypass (CPB) and cardioplegia, a percutaneous prosthesis (17,18) or sutureless valve (19) can be implanted directly into the annulus after excision of the diseased leaflets and decalcification of the annulus. Use of these device may reduce aortic cross-clamp times and provide a greater EOA (after cusp excision) compared with conventional TAVI in patients with a small annulus.
It is promising for the future of transcatheter bioprostheses that their hemodynamic performance(including EOA) is comparable to stentless valves and superior to stented prostheses of the same size (5,20).
Valve-in-valve procedure and PPM
TAVI is also a new therapeutic option for elderly highrisk patients with degenerated aortic valve bioprostheses (21-24). Valve-in-valve (V-in-V) implantation can be performed as a palliative intervention to increase the EOA and decrease valvular regurgitation of degenerated bioprostheses without redo cardiac surgery. However, PPM occurs more frequently than with primary TAVI (25). Appropriate patient selection, and use of manufacturer data on the inner diameter of the surgically implanted valve and the actual echocardiographic measurements of this diameter are crucial. Valve implantation and anchoring are possible in stented and stentless bioprostheses (26). Severe PPM is likely to significantly decrease short- and mid-term survival in patients with valve-in valve-implantation. To date, only Seiffert et al. (25) have addressed severe PPM and its incidence in a small group of V-in-V patients Severe PPM was evident in 5 from 11 patients. There was 1 PPM related surgical intervention.
Conclusions
In common with sAVR, PPM is emerging as an important risk factor for survival following TAVI. The measurement of iEOA is a reliable method of quantifying PPM and may facilitate risk stratification post TAVI.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation 1978;58:20-4.
- Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 2006;92:1022-9.
- Dumesnil JG, Pibarot P. Prosthesis-patient mismatch: an update. Curr Cardiol Rep 2011;13:250-7.
- Rao V, Jamieson WR, Ivanov J, et al. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation 2000;102: III5-9.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;53:1883-91.
- Jilaihawi H, Jeilan M, Spyt T, et al. Early regression of left ventricular wall thickness following percutaneous aortic valve replacement with the CoreValve bioprosthesis. J Invasive Cardiol 2009;21:151-5; discussion 156-8.
- Kukucka M, Pasic M, Dreysse S, et al. Patient-prosthesis mismatch after transapical aortic valve implantation: Incidence and impact on survival. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Hilderman RH, Fairbank AT. Binding and internalization of p1, p4-diadenosine 5'-tetraphosphate by bovine aortic endothelial cells. Biochimie 1999;81:255-60.
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014; quiz 1082-4.
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41.
- Blais C, Dumesnil JG, Baillot R, et al. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003;108:983-8.
- Rabus MB, Kirali K, Kayalar N, et al. Effects of patientprosthesis mismatch on postoperative early mortality in isolated aortic stenosis 2009;18:18-27.
- Jilaihawi H, Chin D, Spyt T, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the Medtronic-Corevalve bioprosthesis. Eur Heart J 2010;31:857-64.
- Pasic M, Unbehaun A, Dreysse S, et al. Rupture of the device landing zone during transcatheter aortic valve implantation: a life-threatening but treatable complication. Circ Cardiovasc Interv 2012;5:424-32.
- Unbehaun A, Pasic M, Dreysse S, et al. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J Am Coll Cardiol 2012;59:211-21.
- Kalavrouziotis D, Rodés-Cabau J, Bagur R, et al. Transcatheter aortic valve implantation in patients with severe aortic stenosis and small aortic annulus. J Am Coll Cardiol 2011;58:1016-24.
- Olsen LK, Arendrup H, Engstrøm T, et al. When operable patients become inoperable: conversion of a surgical aortic valve replacement into transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg 2009;9:837-9.
- Pasic M, Buz S, Unbehaun A, et al. Transcatheter aortic valve implantation combined with conventional heart surgery: Hybrid approach for complex cardiac pathologic features. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Shrestha M, Folliguet T, Meuris B, et al. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis 2009;18:698-702.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Walther T, Falk V, Dewey T, et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol 2007;50:56-60.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-invalve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848-57.
- Pasic M, Unbehaun A, Dreysse S, et al. Transapical aortic valve implantation after previous aortic valve replacement: clinical proof of the “valve-in-valve” concept. J Thorac Cardiovasc Surg 2011;142:270-7.
- Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 2). JACC Cardiovasc Interv 2011;4:733-42.
- Seiffert M, Conradi L, Baldus S, et al. Impact of patientprosthesis mismatch after transcatheter aortic valve-invalve implantation in degenerated bioprostheses. J Thorac Cardiovasc Surg 2012;143:617-24.
- Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 2). JACC Cardiovasc Interv 2011;4:733-42.





