The learning curve associated with transapical aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) has evolved to an accepted treatment option for high-risk elderly patients suffering from severe symptomatic aortic valve stenosis over the past few years. Recently, the first randomized trials demonstrated superiority of TAVI over medical treatment including balloon valvuloplasty (1) and non-inferiority compared to conventional aortic valve replacement (AVR) (2). TAVI prostheses can be implanted using the antegrade transapical (TA) approach or using a retrograde trans-vascular access (transfemoral TF, trans-subclavian TS, trans-aortic Tao). At present, there is no evidence proving the superiority of one or the other approach. Thus, the optimal access should be tailored to the individual patient profile.
Given the truly minimally invasive nature of the TAVI technique, broadening of indications to patients with less risk profiles (moderate risk cohort) is tempting. However, several shortcomings of the current TAVI technique (occurrence of paravalvular leaks, relatively high pacemaker rates, unclear durability etc.) would have to be overcome first, especially in the context of the known excellent results of conventional AVR even in elderly patients (3).
At present, the numbers of T-AVI procedures are rapidly increasing worldwide with several centers just about to start a new TAVI program. Thus, the learning curve in general associated with this new technique and specific major contributing factors are of particular interest.
Methods
To assess the learning curve a total of 299 patients who underwent transapical aortic valve implantation (TA-AVI) using the Edwards SAPIEN™ transcatheter xenograft (Edwards Lifesciences Inc., Irvine, CA, USA) from February 2006 until January 2010 were included in the analysis. The majority of patients in the first half [1-150] were treated within an initial feasibility and then a pivotal study (February 2006 until April 2008). The second half of patients was exclusively treated after CE-approval of the SAPIEN™ device for TA-AVI [151-299]. Follow-up was 100% complete and consisted of a total of 338 patient-years. Detailed results of this analysis have been published recently (4).
Indication for TA-AVI was verified within an interdisciplinary “Heart Team” consisting of cardiologists and cardiac surgeons. Patients were eligible in case of older age (≥75 years) and high risk profile defined by increased risk scores (STS score and/or EuroSCORE I) or in case of other comorbidities suggesting increased surgical risk (liver failure, porcelain aorta, etc.) not reflected by current risk scores.
All procedures were performed in a fully equipped “hybrid OR” by an interdisciplinary team consisting of specialized cardiac anaesthetists, cardiac surgeons and interventional cardiologists. TA-AVI was performed in a standard fashion as described previously (5). A full cardiopulmonary bypass circuit (CPB) was on standby in all cases with femoral wires in place “Safety-net” (6) in all patients to allow for immediate conversion to CPB or conventional surgery if required.
Results
All TA-AVI procedures have been performed by the same team. Preoperative patient variables are shown in Table 1. Patients demonstrated a consistently high risk profile throughout the study indicating that there was no broadening of indication yet. Distribution of cardiac risk factors showed minor variation between groups explaining the slightly lower STS score within the later patients while logistic EuroSCORE I was slightly higher in the second half of patients (Table 2).
| Table 1 Patients demographics | |||
| Patients 1-150 | Patients 151-299 | P | |
|---|---|---|---|
| Age [years] | 82.5±5.7 | 81.8±7.0 | n.s. |
| Female | 104 (69%) | 105 (71%) | n.s |
| NYHA
|
7 (4.7%)
|
40 (26.8%)
|
<0.001 |
| Coronary artery disease * | 65 (43.3%) | 94 (63.1%) | 0.001 |
| Previous cardiac surgery | 31(20.7%) | 55(36.9%) | 0.002 |
| Mitral regurgitation
|
35 (23.3%)
|
60 (40.3%)
|
0.283 |
| LVEF
|
55.4±14.6
|
55.1±12.3
|
0.828
|
| COPD | 64(43%) | 65(44%) | n.s. |
| Pulmonary hypertension | 40 (27%) | 41(28%) | n.s. |
| Peripheral vascular disease | 59 (39.3%) | 83 (55.7%) | 0.005 |
| Carotid artery stenosis ≿0% # | 39(26.0%) | 38(25.5%) | 1.000 |
| Neurological dysfunction | 31(21%) | 25(17%) | n.s. |
| Porcelain aorta | 18 (12.0%) | 21(14.1%) | 0.611 |
| Note: NYHA, New York Heart Association functional class; LVEF, left ventricular ejection fraction; *without hemodynamic relevance, lesions ≤70% or previously revascularized by percutaneous coronary intervention or coronary artery or bypass grafting; #all clinically asymptomatic; *pulmonary artery systolic pressure ≥60 mmHg; COPD, chronic obstructive pulmonary desease/td> | |||
| Table 2 Risk scores | |||
| Risk Scores | Patients 1-150 | Patients 151-299 | P |
|---|---|---|---|
| Log. EuroSCORE I [%] | 29.4±14 | 33.2±17.2 | 0.039 |
| Add. EuroSCORE I | 11.6±1.9 | 12.1±2.5 | n.s. |
| STS Score [%] | 13.5±7.8 | 11.4±7.5 | 0.019 |
| STS: Society of thoracic surgeons | |||
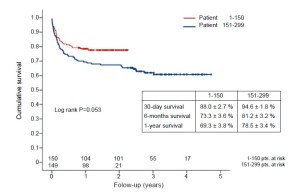
Intraprocedural and postoperative outcomes are shown in Table 3,4. Major stroke rate was extremely low with two patients (0.7%) in total only (Table 5). Overall, there was an obvious trend towards lower 30-day mortality (11.3% vs. 6.0%) and a significant improvement in 1-year survival despite comparable risk profiles (Table 5). The Kaplan-Meier curve (Figure 1) demonstrates the survival of the two patients groups.
| Table 3 Intraoperative variables | |||
| Patients 1-150 | Patients 151-299 | P | |
|---|---|---|---|
| Aortic annulus diameter [mm] | 22.6±1.3 | 22.8±1.6 | n.s. |
| Valve size - mean | 25.2±1.3 | 25.2±1.6 | n.s. |
| Oversizing [mm] | 2.61±0.92 | 2.38±1.01 | 0.041 |
| Device success* | 138 (92%) | 137 (91%) | n.s. |
| Postdilatation | 22 (15%) | 6 (4%) | 0.001 |
| Valve in valve | 6 (4%) | 11 (7%) | n.s. |
| Coronary obstruction** | 7 (5%) | 3 (2%) | n.s. |
| CPB support | 22 (15%) | 8 (5%) | 0.011 |
| Annular tear | 2(2%) | - | - |
| Conversion to sternotomy# | 5 (3%) | 2 (1%) | n.s. |
| Contrast dye [mL] | 104±78 | 93±46 | 0.016 |
| Fluoroscopy [min.] | 7.1±3.9 | 6.2±3.2 | 0.032 |
| Extra stitch at apex | 34 (23%) | 10 (7%) | 0.023 |
| Total OR time [min.] | 94±56 | 84±34 | 0.057 |
| CPB: Cardiopulmonary bypass; *according to VARC definitions; **requiring intervention; # sternotomy, conventional surgery | |||
| Table 4 Postoperative outcome | |||
| Patients 1-150 | Patients 151-299 | P | |
|---|---|---|---|
| Postop hemofiltration | 25 (16.7%) | 22 (14.7%) | n.s. |
| Discharged with new onset chronic dialysis | 2 (2%) | 3 (2%) | n.s. |
| New postoperative pacemaker (AVB III) | 2 (1%) | 8 (5.4%) | n.s. |
| Bleeding requiring rethoracotomy | 3 (2%) | 1 (1%) | n.s. |
| Ventilation time [h]# | 4 | 3.9 | n.s. |
| Extubation ≿4 h | 130 (87%) | 122 (82%) | n.s. |
| Re-Intubation | 26 (17%) | 24 (16%) | n.s. |
| AVB III: Atrio-ventricular conduction block grade three; # median | |||
| Table 5 Mortality and stroke | |||
| Patients 1-150 | Patients 151-299 | P | |
|---|---|---|---|
| Stroke (30-day)* | - | 2(1.3%) | - |
| 30-day mortality | 17 (11.3%) | 9 (6.0%) | 0.77 |
| 1-year mortality | 46 (30.7%) | 32 (21.5%) | 0.047 |
| *Both on postoperative day 1 with arm weakness and both with good functional recovery | |||
A “technical” learning-curve (positioning and implantation) was identified by significantly shorter fluoroscopy time, less contrast dye, less frequent post-implantation balloon re-dilatation and less frequent CPB support with a trend towards shorter total procedure times in the second half of patients.
In addition, residual bleeding requiring extra stiches at the apical access site occurred significantly less frequently over time suggesting a learning curve in regard to the surgical access as well.
Echocardiographic results at discharge are shown in Table 6. Most importantly, the rate of paravalvular leak (mild/moderate) significantly decreased in the second half of patients. Statistically, mean gradients were significantly higher in the more recent patients most likely explained by slightly less oversizing and less frequent re-ballooning. However, clinically this finding is irrelevant as mean gradients are still single digits. Despite apical access the left-ventricular ejection fraction remained stable.
Overall we did not experience any significant problems with the apical access at all.
| Table 6 Postoperative echocardiographic results | |||
| Patients 1-150 | Patients 151-299 | P | |
|---|---|---|---|
| LVEF [%] | 55±13 | 56±11 | n.s. |
| delta preop LVEF [%] | +1.0 | +0.1 | n.s. |
| AV Pmax [mmHg] * | 15±6 | 17±6 | 0.004 |
| AV Pmean [mmHg] | 7.9 ±4 | 9.2±3 | 0.04 |
| AI
(paravalvular leak)
|
72 (48%)
|
102 (68%)
|
0.024 |
| *complete Bernoulli equation; LVEF, left ventricular ejection fraction; delta preoperative LVEF, difference between pre- and postoperative LVEF; Pmax, maximum transvalvular (aortic) pressure gradient; Pmean, mean transvalvular (aortic) pressure gradient; AI, aortic insufficiency (paravalvular leaks) | |||
Discussion
The major conclusion of the data presented is the fact that there is a significant learning curve associated with TA-AVI. Overall, shorter procedure and fluoroscopy times clearly suggest improved handling of devices over time (“wire skills”). In addition, additional stiches at the apical access site were required less frequently after some experience indicating a “surgical” learning curve as well. However, even in the beginning the rate of relevant apical complications was extremely low (<2%) indicating that the transapical access is very safe in general. This is in accordance with recently published data from the multicenter PREVAIL study on transapcial implantation of the SAPIEN-XT™ valve in 150 patients at 12 European sites: There was one complication (1/150=0.7%) (7). Thus the TA access is standardized and safe.
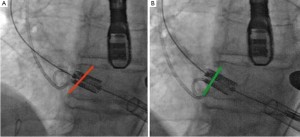
In addition to the typical learning curve associated with all new surgical techniques several specific improvements of the procedure had been developed after an initial pioneering phase. Although these improvements can hardly been shown statistically to be effective we would like to share some of the major refinements. Initially, the SAPIENTM prostheses were positioned under fluoroscopy and angiographic visualization followed by rapid balloon-inflation under rapid ventricular pacing (RVP) which resulted in a kind of “blind” and unpredictable implantation (see Video 1). After some experience, we advocated a “step-wise” implantation technique that allows for final positional adjustments after the balloon is already inflated to 50%. We used this implantation strategy in January 2008 for the first time. In addition, we now perform the implantation with a final angiography after the cardiac output is already diminished by RVP which results in a situation that allows implantation in a “step-wise” manner into a fully contrasted root (see Video 2). Another refinement solved tilting of the SAPIENTM prosthesis in regard to the annular plane (non-axial) especially in case of a “horizontal” aorta by adjusting the wire-slack (Figure 2). Altogether we believe that using these techniques and the very short and direct transapical access valves can be implanted with utmost precision which might have contributed to a significantly lower rate of paravalvular leaks in the second half of patients.
Another refinement is in regard to the management of hemodynamic collapse which occurs rarely, but if present either secondary due to complications (coronary occlusion, severe leak) or after RVP in patients with reduced ventricular function. Prior to skin incision a femoral venous guidewire is placed and advanced to the SVC under fluoroscopy. Together with the femoral arterial sheath (needed for root angiography) this setup allows for rapid percutaneous cannulation and bail-out to CPB (“Safety-Net”) (6). In addition, direct injection of diluted epinephrine (small boluses of 10 or 20 µg) into the aortic root over the pigtail worked very well in our experience to immediately restore circulation in case hemodynamic depression after valve implantation occurs.
When trying to assess the learning curve with TA-AVI today the problem is to discriminate the “individual” (center or operator specific) from the “global” (worldwide) learning curve as now most new centers will start off with proctored cases and much more experience has been gathered compared to the early experiences included in the data presented herein (8). In addition, the SAPIENTM device has undergone improvement regarding the valve itself (new SAPIENTM XT) (7) and the delivery system (new AscendraTM II). Furthermore, new transapical devices are available in Europe which might ease the learning curve at least in regard to positioning (9).
Another area that underwent major improvements is the better understanding of imaging modalities for TA-AVI. Enhanced imaging techniques are of utmost help for optimal C-arm angulation (perpendicularity) (10) and recently it became apparent that sizing T-AVI valves based on MSCT (effective diameter: area or perimeter) is more precise than TEE (11,12).
When trying to compare current T-AVI outcomes the shortcoming is the lack of a reliable T-AVI risk score (13,14) which optimally would include “frailty” (15) as well as patient specific valve anatomy (severity of calcification, calcification pattern) (16). In addition, results from highly selected patients in randomized US trials (1,2) are hardly comparable to European “all-comers” registries (17,18).
Conclusions
Today, TA-AVI has evolved to a routine procedure to treat severe symptomatic aortic stenosis in elderly highrisk patients in specialized high-volume centers. After an initial pioneering phase several refinements have led to a stabilization of results. Although a learning curve has to be overcome first, as with all new surgical techniques, a dedicated interdisciplinary team will be quickly able to establish this new procedure. Due to the vast experience gathered worldwide, new centers will be able to safely start a new TA-AVI program given the excellent training and proctoring programs available today.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Vasques F, Messori A, Lucenteforte E, et al. Immediate and late outcome of patients aged 80 years and older undergoing isolated aortic valve replacement: a systematic review and meta-analysis of 48 studies. Am Heart J 2012;163:477-85.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011;124:S124-9.
- Walther T, Dewey T, Borger MA, et al. Transapical aortic valve implantation: step by step. Ann Thorac Surg 2009;87:276-83.
- Kempfert J, Walther T, Borger MA, et al. Minimally invasive off-pump aortic valve implantation: the surgical safety net. Ann Thorac Surg 2008;86:1665-8.
- Walther T, Thielmann M, Kempfert J, et al. PREVAIL TRANSAPICAL: multicentre trial of transcatheter aortic valve implantation using the newly designed bioprosthesis (SAPIEN-XT) and delivery system (ASCENDRA-II). Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Wendler O, Walther T, Schroefel H, et al. The SOURCE Registry: what is the learning curve in trans-apical aortic valve implantation? Eur J Cardiothorac Surg 2011;39:853- 9; discussion 859-60.
- Kempfert J, Rastan AJ, Beyersdorf F, et al. Trans-apical aortic valve implantation using a new self-expandable bioprosthesis: initial outcomes. Eur J Cardiothorac Surg 2011;40:1114-9.
- Kempfert J, Noettling A, John M, et al. Automatically segmented DynaCT: enhanced imaging during transcatheter aortic valve implantation. J Am Coll Cardiol 2011;58:e211.
- Kempfert J, Van Linden A, Lehmkuhl L, et al. Aortic annulus sizing: echocardiographic vs. computed tomography derived measurements in comparison with direct surgical sizing. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre realworld registry. Eur Heart J 2011;32:198-204.
- Dewey TM, Brown D, Ryan WH, et al. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008;135:180-7.
- Gummert JF, Funkat A, Osswald B, et al. EuroSCORE overestimates the risk of cardiac surgery: results from the national registry of the German Society of Thoracic and Cardiovascular Surgery. Clin Res Cardiol 2009;98:363-9.
- Sündermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg 2011;39:33-7.
- Haensig M, Lehmkuhl L, Rastan AJ, et al. Aortic valve calcium scoring is a predictor of significant paravalvular aortic insufficiency in transapical-aortic valve implantation. Eur J Cardiothorac Surg 2012;41:1234-40; discussion 1240-1.
- Wendler O, Walther T, Schroefel H, et al. Transapical aortic valve implantation: mid-term outcome from the SOURCE registry. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7.





