A systematic review of transapical aortic valve implantation
Background: Transcatheter aortic valve implantation (TAVI) through a transapical approach (TAAVI) for
severe aortic stenosis becomes the procedure of choice in cases where patients have peripheral artery disease
and unfeasible access due to excessive atherosclerotic disease of the iliofemoral vessels and aorta. The present
systematic review aimed to assess the safety, success rate, clinical outcomes, hemodynamic outcomes, and
survival benefits of TAAVI.
Methods: Transcatheter aortic valve implantation (TAVI) through a transapical approach (TAAVI) for
severe aortic stenosis becomes the procedure of choice in cases where patients have peripheral artery disease
and unfeasible access due to excessive atherosclerotic disease of the iliofemoral vessels and aorta. The present
systematic review aimed to assess the safety, success rate, clinical outcomes, hemodynamic outcomes, and
survival benefits of TAAVI.
Results: After applying the inclusion and exclusion criteria, 48 out of 154 shortlisted potentially relevant
articles were selected for assessment. Of these, 26 studies from 24 centers including total number of 2,807
patients were included for appraisal and data extraction. The current evidence on TAAVI for aortic stenosis is
limited to observational studies. Successful TAAVI implantation occurred in >90% of patients. On average,
the procedure took between 64 to 154 minutes to complete. The incidence of major adverse events
included 30-day mortality (4.7-20.8%); cerebrovascular accident (0-16.3%); major tachyarrhythmia
(0-48.8%); bradyarrhythmia requiring permanent pacemaker insertion (0-18.7%); cardiac tamponade
(0-11%); major bleeding (1-17%); myocardial infarction (0-6%); aortic dissection/rupture (0-5%); moderate
to severe paravalvular leak (0.7-24%); cardiopulmonary bypass support (0-15%); conversion to surgery
(0-9.5%); and valve-in-valve implantation (0.6-8%). Mean aortic valve area improved from 0.4-0.7 cm2
before TAAVI to 1.4-2.1 cm2 after TAAVI. The peak pressure gradient across the aortic valve decreased
from >70 mmHg to <20 mmHg after TAAVI. One-year survival ranged from 49.3% to 82% and the 3-year
survival was 58% in 2 series.
Conclusions: TAAVI appears to be feasible with a reasonable safety and efficacy portfolio. Randomised
controlled trials are required to compare transapical vs. transfemoral TAVI when both techniques are equally feasible.
Key words: Transcatheter aortic valve implantation (TAVI); systematic review; aortic stenosis (AS)
Introduction
Aortic stenosis (AS) is the most common valve disease, and once symptomatic can lead to a decrease in life expectancy (1). Aortic valve replacement (AVR) has long been the definitive therapy in treating symptomatic AS. However, in the face of an increasingly older population and increasing prevalence of AS, a percentage of patients may not be deemed candidates for surgery due to high surgical risk or other prohibitive risk factors. Transcutaneous aortic valve implantation (TAVI) has become an accepted alternative to surgery in treating severe AS for high-risk or non-operative individuals (2).
TAVI can be performed using several approaches including a retrograde transfemoral (TFAVI), transsubclavian, transaortic, or antegrade transapical. The TFAVI approach is often considered the first choice for TAVI due to its minimal invasiveness and reduced anaesthetic requirement (3). Transapical TAVI (TAAVI) becomes the procedure of choice in instances where patients have excessive atherosclerotic disease of the iliofemoral vessels and aorta, and peripheral access is not feasible. For TAAVI, the balloon-expandable Edwards SAPIEN (Edwards Lifesciences, Irvine, CA) prosthesis with the Ascendra delivery system gained CE (European Conformity) mark approval in 2008. Thereafter, CE mark approvals were granted to the second-generation Edwards SAPIEN XT prosthesis (23-mm and 26-mm valves) and the Ascendra II delivery system in 2010 and the SAPIEN XT 29-mm prosthesis in 2011. Several other devices from different companies (Jenavalve, Jena Valve Inc, Munich, Germany; Embracer, Medtronic Inc, Guilford, CT; Accurate, Symetis Inc, Geneva, Switzerland) have passed “first in man trials” successfully and are being evaluated within multicenter studies (4). The subclavian artery is the other alternative access route when a transfemoral approach is not feasible. The self-expanding CoreValve ReValving system (Medtronic, Minneapolis, MN) is another commonly used valve that can be delivered retrogradely via both transfemoral and trans-subclavian approaches (5).
Despite the growing number of patients undergoing TAAVI each year, there is no comprehensive review assessing the safety and efficacy of this approach. We performed this systematic meta-analysis and review to assess the safety, success rate, clinical outcomes, hemodynamic outcomes, and survival benefits of TAAVI.
Methods
Literature search strategy
A systematic review was performed and six electronic databases including MEDLINE, EMBASE, PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Database of Abstracts of Review of Effectiveness were searched for original published studies from January 2000 to February 2012. To achieve the maximum sensitivity of the search strategy and identify all studies, we used appropriate free text and thesaurus terms: “percutaneous” OR “transcutaneous” OR “transcatheter” OR “transarterial” OR “transapical” AND “aortic valve” OR “aortic valve stenosis”. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies.
Outcome measures
The primary end points included feasibility and safety (procedural success rate, 30-day mortality, major tachyarrhythmia, bradyarrythmia requiring permanent pacemaker insertion, myocardial infarction, cardiac tamponade, cerebrovascular accident, conversion to surgery, moderate to severe paravalvular leak, valve-in-valve procedure, emergency percutaneous coronary intervention, aortic dissection/perforation, major bleeding, procedure and fluoroscopy duration, and length of hospital stay). The secondary outcomes included echocardiographic findings (mean aortic valve area before and after TAAVI, peak and mean pressure gradient before and after TAAVI, left ventricular ejection fraction before and after TAAVI), New York Heart Association [NYHA] functional class improvement versus baseline, and survival at 6-month, 1-year, 2-year, and 3-year follow-up reviews.
Selection criteria
Studies eligible for this systematic review included highrisk patients with AS who received TAAVI using the Edwards SAPIEN transcatheter xenograft(Edwards Lifesciences, Irvine, CA). The criteria for patient selection for TAAVI varied among institutions, and the definitions for nonsurgical candidates were not uniform. Experimental or observational studies were included in the present review. Case reports, series with less than ten patients, abstracts, editorials, and expert opinions were excluded. Case series limited to a selected group of patients (redo surgeries, valvein- valve implantation, etc.) were excluded.
Serial publications reporting accumulating numbers of patients or increased length of follow-up were identified. The publication with the most complete data set from each center was retained. Data was extracted from two papers from each of the two centers that had competing data on the same patient population (6-9).
Data extraction and critical appraisal
Two reviewers (M.R. and J.S.) independently appraised each included study using a standard form and extracted data on methodology, quality criteria, and outcome measures. All data was extracted and tabulated from the relevant articles’ texts, tables, and figures. The quality of studies was assessed using criteria recommended by the National Health Service Centre for Reviews and Dissemination case series quality assessment criteria (University of York, Healington, United Kingdom) (10). Clinical effectiveness was synthesized through a narrative review with full tabulation of results of all included studies. Discrepancies between the 2 reviewers were resolved by discussion and consensus with a third investigator (T.D.Y.).
Intervention
Despite some variations, similar steps are followed in various centers. The operative technique for TAAVI is well described in the literature (11). In brief, an anterolateral mini-thoracotomy is performed in the fifth intercostal space. After pericardiotomy, the left ventricular apex is punctured between two pledgeted purse-string sutures. Balloon valvuloplasty of the stenotic valve is then performed under rapid ventricular pacing. Under guidance of fluoroscopy and transesophageal echocardiography, the valve is then positioned within the aortic annulus and implanted during a second period of rapid ventricular pacing.
Results
Quantity of studies
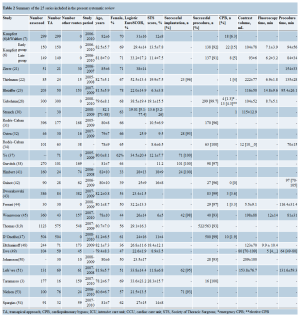
After removing duplicates, the titles and abstracts of 671 peerreviewed publications were identified through searching the 6 electronic databases. Initial evaluation of these abstracts identified 151 potentially relevant publications. Manual search of the reference lists identified 3 additional publications of interest. When the inclusion and exclusion criteria were applied to these 154 publications, 48 articles (3,6-9,12-54) remained for assessment (Table 1). In total, 25 series presented in 27 studies (3,6-9,21-23,28,30-32,34,37,38,41-45,47-51,53,54) including total number of 2,807 patients were included for appraisal and data extraction (Table 2).
Quality of evidence
No randomized controlled trials were identified. Papers presented data on groups of patients who had undergone TFAVI or TAAVI (3,8,9,22,23,31,32,34,38,41-43,45,48- 51,53,54), TAAVI only (6,7,28,30,37,44,47), or compared between TAAVI and open AVR (21). All reports originated from specialized tertiary referral centers. Seven centers reported results of TAAVI in 100 or more (range, 101-575) patients (6-9,28,30,31,38,47). There were five multicentric series (8,31,47,48,51).
Twelve studies reported explicit inclusion criteria (6,7,28,32,41-44,47,48,51,54). The definitions of highrisk patients with AS not suitable for surgical AVR varied among the institutions; for example, age >75 years (6,21); NYHA functional class II or more (48,51); AVA 2 (32,45,48), 2 (21,47,51,54); logistic EuroSCORE >20% (9,21,28,41-44,47,48,51,54), logistic EuroSCORE >15% (45), additive EuroSCORE ≥9 (6,7), and/or Society of Thoracic Surgeons score >10% (6,28,41-43,48,51,54). Operative technique was clearly explained in ten studies (21,23,32,37,41-44,48,51). Procedures were performed in either a surgical hybrid suite (7,22,23,28), an angiography suite (21,42,53,54), or in the operating theater (32,37,42,44). The definitions of adverse events were clearly explained in 8 studies (9,32,38,41,42,45,47,51).
Assessment of feasibility
Success of the procedure occurred in >90% of cases in studies that reported this outcome (Table 2). Procedural success rate was 92.7% (522/563) in a multicentric European registry (SOURCE registry), with 20 patients (3.5%) requiring conversion to open AVR (8). Valve-invalve implantation was required in 19 patients (3.3%) in the SOURCE registry to correct malposition or moderate/ severe aortic insufficiency after placement of the first valve (8). D’Onofrio et al. (47) reported successful implantation in 99% (500/504) of patients undergoing TAAVI in an Italian multicentric registry. Valve-in-valve implantation was performed in 3 patients because of malpositioning of the first prosthesis and 1 patient required conversion to an open AVR after the valve embolized to the left ventricle.
Kempfert et al. (6) reported similar device success rates in their first 150 patients (138/150; 92%) compared to the next 149 patients (137/149; 91%) that had undergone TAAVI. Requirement for conversion to AVR or valve-in-valve implantation was similar between earlier and later groups of patients in this series (6).
Assessment of safety
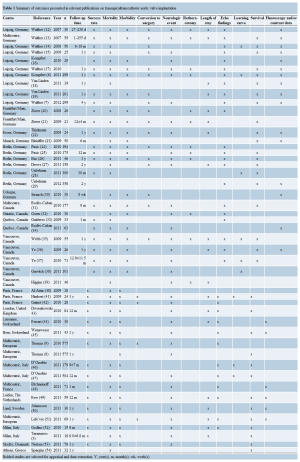
Table 3 summarizes 30-day major adverse events following TAAVI across all studies. The range of these adverse events was as following: 30-day mortality (4.7-20.8%); cerebrovascular accident (0-16.3%); major tachyarrhythmia (0-48.8%); bradyarrhythmia requiring permanent pace maker insertion (0-18.7%); cardiac tamponade (0-11%); major bleeding (1-17%); myocardial infarction (0-6%); aortic dissection/rupture (0-5%); moderate to severe paravalvular leak (0.7-24%); cardiopulmonary bypass support (0-15%); conversion to surgery (0-9.5%), and valve-in-valve implantation (0.6-8%). The procedure took between 64 to 154 minutes on average to complete. The reported mean volume of contrast used varied widely between studies (12- 222 mL) (Table 2). In a series from Leipzig, Germany, less contrast volume, shorter fluoroscopy time and less frequent cardiopulmonary bypass support were required as the procedural team gained experience (6). The mean length of ICU stay varied between 1 to 5 days, while, the mean length of hospital stay ranged from 5 to 15 days (Table 3).
Assessment of efficacy
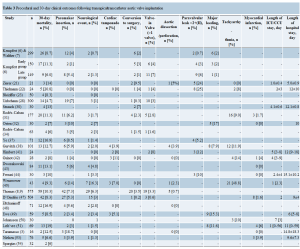
Echocardiographic findings are demonstrated in Table 4. Mean aortic valve area improved from 0.4-0.7 cm2 before TAAVI to 1.4-2.1 cm2 after TAAVI. The peak pressure gradient across the aortic valve decreased from >70 mmHg to <20 mmHg after TAAVI (Table 4). In a few series, symptomatic improvement occurred as evidenced by a decrease in NYHA functional class (Table 5). The number of patients with NYHA functional class III or IV reduced from 71% (42/59) before intervention to 36% (14/39) in 6 months and to 26% (6/23) in 12 months after TAAVI in one series (49).
The multicentric European PARTNER transcatheter heart valve study (51) showed that the frequency of patients with NYHA functional class III or IV symptoms decreased from 85.5% (59/69) before procedure to 14.7% (5/34) one year after TAAVI. When the EuroQol with EQ-5D UKTTO rating scale (not specific for cardiac patients) was used to assess the quality of life, only marginal difference was noted in the one-year follow-up (n=20, 0.59±0.30 baseline vs. 0.66±0.43 one year after TAAVI; P=0.13). However, the Kansas City Cardiomyopathy Questionnaire (KCCQ), a more specific questionnaire for cardiac patients, showed significant improvement in the quality of life in one year (n=23, 49.6±22.7 baseline vs. 77.1±23.4 one year after TAAVI; P=0.0004) (51).
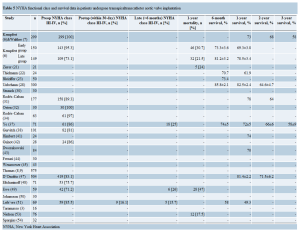
Assessment of survival
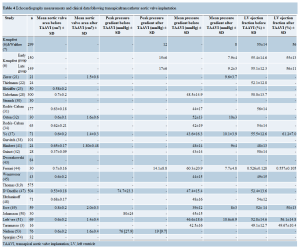
One year survival ranged from 49.3% to 82.5% (Table 5). Mid-term (≥1 year) survival data was recorded in series from 9 centers (Table 5) (6,7,22,28,31,37,41,43,47,51). The 3-year survival was 58% in 2 studies (7,37).
Discussion
The worldwide experience in TAAVI is growing. The current systematic review presents the procedural outcomes of 24 series with a total number of 2,724 patients that have undergone TAAVI worldwide. The procedural success rate ranged from 93% to 100% across all studies that reported this outcome (Table 2). In the largest series included in this review, Thomas et al. (9) reported a 30-day mortality of 10% following TAAVI. The reported 1-year survival was often greater than 70% (Table 5), with Walther et al. (7) reporting a 3-year survival of 58% in 299 patients who had undergone a TAAVI.
Based on echocardiography and NYHA functional class, TAAVI proved to be efficacious with symptomatic improvement at 6- and 12-month follow-up. However, it remains unclear whether there is a correlation between improvement in valvular hemodynamics and patient’s quality of life (2). One study (European PARTNER) was able to demonstrate a significant improvement in the quality of life at 12 months (51), but additional evidence is still needed.
Learning curves play a role in determining an operator’s and institute’s overall outcomes. In their series of 300 TAAVI patients, Unbehaun et al. (28) reported a reduction in overall 30-day mortality from 6% for the first 100 patients to 2% for the last 100 patients. In the same series, the six-month survival rate increased from 84% in the early group to 96% in the late group (28). Similarly, Ye et al. (37) reported a 33.3% mortality in the beginning of their cohort and a 12.5% with the remaining patients. Furthermore, Kempfort et al. reported decreased 30-day mortality rates from 11% in the first 150 patients to 6% in the next 149 patients receiving a TAAVI. In the same series, the 1-year mortality significantly improved from 30.7% to 21.5% between the two groups (P=0.047) (6). Nevertheless, results from the Italian Registry of Trans-Apical Aortic Valve Implantation (I-TA) suggested no significant differences in outcomes between high- and low-volume centers and between the first and the second 50% of cases (47).
Risk factors for mortality proved to be heterogeneous between studies. When multivariate analysis was performed by Kempfort et al., reduced vital capacity (grade 1) were the only independent predictors of 30-day mortality in a series of 299 patients. Of interest, variables such as age, logistic EuroSCORE >30%, and STS score >15% failed to predict mortality in this series (6). While in the I-TA registry, multivariate analysis identified NYHA class III and IV (OR, 4.43; 95%CI, 1.28- 15.40), logistic EuroSCORE >20 (OR, 1.83; 95%CI,1.02- 3.29), creatinine concentration >200 mmol/L (OR, 2.56; 95%CI, 1.07-6.15), and intraoperative complications (OR, 5.80; 95% CI, 2.68-12.55) as independent risk factors for mortality after TAAVI (47).
In a joint position statement published for 2012, the American College of Cardiology Foundation (ACCF) Board of Trustees, American Association for Thoracic Surgery (AATS) Council, Society for Cardiovascular Angiography Interventions (SCAI) Board of Directors, and Society of Thoracic Surgeons (STS) have established a guideline of recommendations in the selection of patients for TAVI, who would be deemed a prohibitive or high surgical risk, yet there are no specific inclusion criteria for TAAVI (55). To our knowledge, there has been no randomized trial reported so far comparing TFAVI versus TAAVI. Despite TAAVI being considered more invasive than TFAVI, preliminary results suggest TAAVI as having less vascular complications, decreased use of contrast or fluoroscopy, and possible different adverse neurologic outcomes. Based on the current literature, TAAVI and TFAVI patients cannot be compared without a significant bias. Ewe et al. (49) highlighted the possibility of such a selection bias, noting that TAAVI patients carried a higher perioperative risk compared to TFAVI patients. Similarly, Eltchaninoff et al. (48) demonstrated that patients treated by TAAVI had more comorbidities than patients selected for TFAAVI in particular more peripheral vascular disease. This invariably increases their mortality risk. Additionally, Nielsen et al. (53) observed TAAVI patients to have a greater burden of comorbidity, reflected in a higher EuroSCORE than that of TFAVI patients (21.5% vs. 15.9%). The SOURCE investigators (8) also highlighted a higher logistic EuroSCORE (29% vs. 25.8%; P=0.007) comparing TAAVI vs. TFAVI.
In conclusion, based on the results available from more than 2,700 patients gathered in this review, TAAVI can be performed with acceptable safety profiles and reasonable survival outcomes. Furthermore, TAAVI can be chosen as the primary access route, although current common practice uses TAAVI as an alternative when TFAVI cannot be safely performed. Randomised controlled trials are required to compare TAAVI vs. TFAVI as standard primary approaches for TAVI when both techniques are equally feasible.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66.
- Yan TD, Cao C, Martens-Nielsen J, et al. Transcatheter aortic valve implantation for high-risk patients with severe aortic stenosis: A systematic review. J Thorac Cardiovasc Surg 2010;139:1519-28.
- Taramasso M, Maisano F, Cioni M, et al. Trans-apical and trans-axillary percutaneous aortic valve implantation as alternatives to the femoral route: short- and middle-term results. Eur J Cardiothorac Surg 2011;40:49-55.
- Walther T, Mollmann H, van Linden A, et al. Transcatheter aortic valve implantation transapical: step by step. Semin Thorac Cardiovasc Surg 2011;23:55-61.
- Petronio AS, De Carlo M, Bedogni F, et al. 2-Year Results of CoreValve Implantation Through the Subclavian Access: A Propensity-Matched Comparison With the Femoral Access. J Am Coll Cardiol 2012. [Epub ahead of print].
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011;124:S124-9.
- Walther T, Kempfert J, Rastan A, et al. Transapical aortic valve implantation at 3 years. J Thorac Cardiovasc Surg 2012;143:326-31.
- Thomas M, Schymik G, Walther T, et al. Oneyear outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011;124:425-33.
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9.
- Undertaking systemic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. York: NHS Centre for Reviews and Dissemination, 2001.
- Walther T, Dewey T, Borger MA, et al. Transapical aortic valve implantation: step by step. Ann Thorac Surg 2009;87:276-83.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation--proof of concept. Eur J Cardiothorac Surg 2007;31:9-15.
- Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008;33:983-8.
- Walther T, Falk V, Borger MA, et al. Transapical aortic valve implantation in patients requiring redo surgery. Eur J Cardiothorac Surg 2009;36:231-4; discussion 4-5.
- Kempfert J, Van Linden A, Linke A, et al. Transapical aortic valve implantation: therapy of choice for patients with aortic stenosis and porcelain aorta? Ann Thorac Surg 2010;90:1457-61.
- Walther T, Schuler G, Borger MA, et al. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 2010;31:1398-403.
- Van Linden A, Kempfert J, Blumenstein J, et al. Transapical aortic valve implantation off-pump in patients with impaired left ventricular function. Ann Thorac Surg 2011;92:18-23.
- Van Linden A, Kempfert J, Rastan AJ, et al. Risk of acute kidney injury after minimally invasive transapical aortic valve implantation in 270 patients. Eur J Cardiothorac Surg 2011;39:835-42; discussion 42-3.
- Zierer A, Wimmer-Greinecker G, Martens S, et al. The transapical approach for aortic valve implantation. J Thorac Cardiovasc Surg 2008;136:948-53.
- Zierer A, Wimmer-Greinecker G, Martens S, et al. Is transapical aortic valve implantation really less invasive than minimally invasive aortic valve replacement? J Thorac Cardiovasc Surg 2009;138:1067-72.
- Thielmann M, Wendt D, Eggebrecht H, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg 2009;88:1468-74.
- Bleiziffer S, Ruge H, Mazzitelli D, et al. Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. J Thorac Cardiovasc Surg 2009;138:1073-80.
- Pasic M, Buz S, Dreysse S, et al. Transapical aortic valve implantation in 194 patients: problems, complications, and solutions. Ann Thorac Surg 2010;90:1463-9; discussion 9-70.
- Pasic M, Unbehaun A, Dreysse S, et al. Transapical aortic valve implantation in 175 consecutive patients: excellent outcome in very high-risk patients. J Am Coll Cardiol 2010;56:813-20.
- Buz S, Pasic M, Unbehaun A, et al. Trans-apical aortic valve implantation in patients with severe calcification of the ascending aorta. Eur J Cardiothorac Surg 2011;40:463-8.
- Drews T, Pasic M, Buz S, et al. Transapical aortic valve implantation after previous heart surgery. Eur J Cardiothorac Surg 2011;39:625-30.
- Unbehaun A, Pasic M, Drews T, et al. Analysis of survival in 300 high-risk patients up to 2.5 years after transapical aortic valve implantation. Ann Thorac Surg 2011;92:1315-23.
- Unbehaun A, Pasic M, Dreysse S, et al. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J Am Coll Cardiol 2012;59:211-21.
- Strauch JT, Scherner MP, Haldenwang PL, et al. Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury. Ann Thorac Surg 2010;89:465-70.
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90.
- Osten MD, Feindel C, Greutmann M, et al. Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the Edwards Sapien balloonexpandable bioprosthesis: a single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv 2010;75:475-85.
- Gutiérrez M, Rodes-Cabau J, Bagur R, et al. Electrocardiographic changes and clinical outcomes after transapical aortic valve implantation. Am Heart J 2009;158:302-8.
- Rodés-Cabau J, Gutierrez M, Bagur R, et al. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol 2011;57:1988-99.
- Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valverelated outcomes. Circulation 2009;119:3009-16.
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: 1-year outcome in 26 patients. J Thorac Cardiovasc Surg 2009;137:167-73.
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg 2010;139:1107-13, 13.e1.
- Gurvitch R, Tay EL, Wijesinghe N, et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv 2011;78:977-84.
- Higgins J, Ye J, Humphries KH, et al. Early clinical outcomes after transapical aortic valve implantation: a propensity-matched comparison with conventional aortic valve replacement. J Thorac Cardiovasc Surg 2011;142:e47-52.
- Al-Attar N, Ghodbane W, Himbert D, et al. Unexpected complications of transapical aortic valve implantation. Ann Thorac Surg 2009;88:90-4.
- Himbert D, Descoutures F, Al-Attar N, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol 2009;54:303-11.
- Guinot PG, Depoix JP, Etchegoyen L, et al. Anesthesia and perioperative management of patients undergoing transcatheter aortic valve implantation: analysis of 90 consecutive patients with focus on perioperative complications. J Cardiothorac Vasc Anesth 2010;24:752-61.
- Dworakowski R, MacCarthy PA, Monaghan M, et al. Transcatheter aortic valve implantation for severe aortic stenosis-a new paradigm for multidisciplinary intervention: a prospective cohort study. Am Heart J 2010;160:237-43.
- Ferrari E, Sulzer C, Marcucci C, et al. Transapical aortic valve implantation without angiography: proof of concept. Ann Thorac Surg 2010;89:1925-32.
- Wenaweser P, Pilgrim T, Roth N, et al. Clinical outcome and predictors for adverse events after transcatheter aortic valve implantation with the use of different devices and access routes. Am Heart J 2011;161:1114-24.
- D’Onofrio A, Fusari M, Abbiate N, et al. Transapical aortic valve implantation in high-risk patients with severe aortic valve stenosis. Ann Thorac Surg 2011;92:1671-7.
- D’Onofrio A, Rubino P, Fusari M, et al. Clinical and hemodynamic outcomes of “all-comers” undergoing transapical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation (I-TA). J Thorac Cardiovasc Surg 2011;142:768-75.
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7.
- Ewe SH, Delgado V, Ng ACT, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg 2011;92:1244-51.
- Johansson M, Nozohoor S, Kimblad PO, et al. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011;91:57-63.
- Lefèvre T, Kappetein AP, Wolner E, et al. One year followup of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J 2011;32:148-57.
- Godino C, Maisano F, Montorfano M, et al. Outcomes after transcatheter aortic valve implantation with both Edwards-SAPIEN and CoreValve devices in a single center: the Milan experience. JACC Cardiovasc Interv 2010;3:1110-21.
- Nielsen HH, Thuesen L, Egeblad H, et al. Single center experience with transcatheter aortic valve implantation using the Edwards SAPIENTM Valve. Scand Cardiovasc J 2011;45:261-6.
- Spargias K, Polymeros S, Dimopoulos A, et al. The predictive value and evolution of N-terminal pro-B-type natriuretic peptide levels following transcutaneous aortic valve implantation. J Interv Cardiol 2011;24:462-9.
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/ SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collaboration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Ann Thorac Surg 2012;93:1340-95.