Preoperative patient optimization for mechanical circulatory support
Introduction
Two patients are presented for consideration of left ventricular assist device (LVAD) placement for mechanical circulatory support. Pertinent right heart catheterization and laboratory data are presented in Table 1.
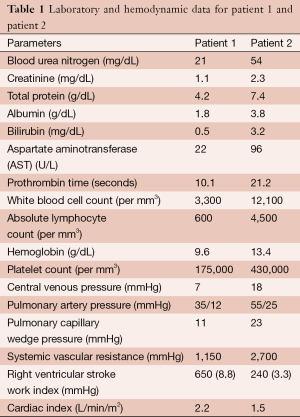
Full table
- A 56-year-old man with ischemic cardiomyopathy with a left ventricular ejection fraction of 20%. He has had five admissions in the last six months for intravenous loop diuretics, despite dietary and medication compliance and optimization of his outpatient medical regimen. Body mass index (BMI) is 18;
- A 42-year-old woman presents from home with lethargy after months of “feeling sick”. On admission, blood pressure is 72/58 mmHg, heart rate is 118 beats/minutes, and oxygen saturation is 84% on room air. She is intubated and transferred to the cardiac intensive care unit, where transthoracic echocardiogram shows a dilated left ventricle with an ejection fraction of 15% and global hypokinesis of both ventricles.
Both of these patients raise unique questions of how the team can best optimize their risk prior to LVAD placement in order to avoid foreseeable postoperative pitfalls. There are three common postoperative complications in LVAD patients and established pre-operative predictors and preventative strategies for each.
Prior to discussing those complications, it is important to review the contraindications for LVAD placement (Table 2) (1). While these may change as technology advances, currently, the presence of any of these contraindications should obviate LVAD placement. It is also important to recognize that some contraindications are reversible and short term mechanical support can occasionally be considered in these patients.
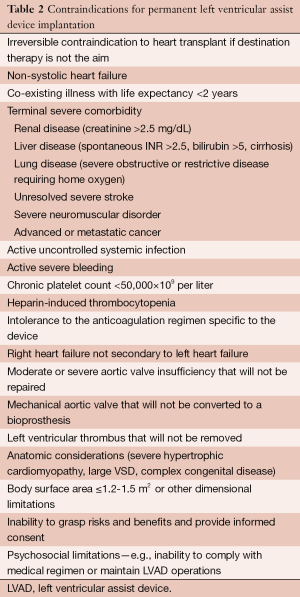
Full table
Safeguards and pitfalls
Postoperative pitfall 1: right ventricular (RV) failure
In the early post-operative period, several mechanisms may contribute to RV failure, including a sudden increase in cardiac output, increased RV preload from increased venous return, and increased RV wall strain and afterload due to septal shift, and pulmonary vasoreactivity after cardiopulmonary bypass. RV failure leads to liver and renal failure, underfilling of the LV and pump causing arrhythmias or shock, and a higher peri-operative mortality (1). Table 3 lists independent clinical, laboratory, hemodynamic, and echocardiographic predictors of RV failure.
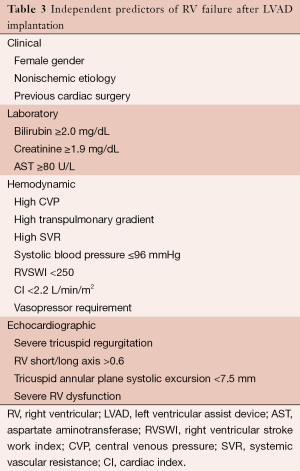
Full table
Prospective data is limited regarding pre-operative interventions (aside from diligent patient selection) that effectively reduce the risk of RV failure. Anecdotal evidence and experience has suggested that pre-operative diuresis, use of intra-aortic balloon pump, and inotropic support to optimize RV preload and hemodynamics can reduce the risk of RV failure in high risk patients (1).
Postoperative pitfall 2: bleeding
In patients undergoing placement of continuous flow devices, the most common postoperative adverse event in the first 30 days is nonsurgical bleeding (2). The reported incidence of nonsurgical bleeding ranges from 15-53% in the literature (2,3), with studies of newer centrifugal flow devices reporting lower bleeding rates (13-15%) (4). The most common sources of bleeding are mediastinal and thoracic (high early risk, but diminishing over time), epistaxis, intracranial, and gastrointestinal (GI) tract (more prominent later after implantation, with an average time to first GI bleed of 460 days) (2,5). The risk of GI bleeding is higher with continuous-flow than with pulsatile-flow devices, and also higher than would be expected from the anticoagulation required for the continuous-flow devices (which is not required in the pulsatile-flow devices) (6). Proposed mechanisms include the loss of high-molecular-weight von Willebrand factor induced by increased shear stress in axial-flow devices (which is known to occur in the first 30 days after implantation), angiodysplasia formation, and impaired platelet aggregation (5,6).
Again, careful patient selection is crucial to mitigating postoperative nonsurgical bleeding. The Model for End-Stage Liver Disease (MELD) score has been found to identify LVAD candidates at high risk for perioperative bleeding (7). This score incorporates bilirubin, creatinine, and prothrombin time, factors which should be included in any pre-operative screen in patients considered for LVAD placement. Hematocrit, platelet count and aggregation studies, and partial thromboplastin time should also be checked. In addition, improving nutrition (particularly vitamin K supplementation), and to the extent possible, hepatic function can reduce the risk of peri-operative bleeding (7). Preoperatively, one should try to aggressively diurese to decongest the liver, which anecdotally appears to decrease the risk of bleeding. Post-operatively, considering holding all anticoagulation for a few days is reasonable and does not appear to increase the risk of pump thrombus. Care should also be taken in the post-operative period to tightly control anticoagulation levels and educate patients about the dietary and monitoring requirements of systemic anticoagulation therapy (8).
Postoperative pitfall 3: infection
Post-implantation sepsis and device-related infections remain inherent risks in patients undergoing LVAD implantation. Depending on the population, between 19% and 48% of patients suffer a device-related infectious complication (2,9). In one trial, sepsis accounted for more than twice the number of deaths than device failure (41% vs. 17%) (10). Risk factors for developing infection can be divided into device-related factors (larger device size, driveline caliber), operative factors (longer surgical times, increased bleeding, failure to provide operative antibiotics), and patient-related factors (9). While numerous studies have failed to identify age, gender, race, and even the presence of diabetes as patient-related risk factors for infection, there is substantial data suggesting that malnutrition is associated with increased risk (9,11).
Heart failure patients are at risk for malnutrition through multiple mechanisms—anorexia, early satiety, delayed gastric emptying, and a chronic inflammatory state all contribute to cardiac cachexia (12). Serologic markers of nutritional status (low serum albumin, total protein, and absolute lymphocyte count) are associated with adverse clinical outcomes, including post-implantation sepsis (11).
What remains less clear is our ability to diminish a malnourished patient’s risk with enteral or parenteral nutritional supplementation. Several studies have documented reduced mortality in patients with higher pre-implantation BMI—the so-called “obesity paradox”—with patients with a BMI <20 accruing the highest risk (12). Thus, it is reasonable to consider nutrition supplementation in patients with high risk serologic markers of malnutrition and a low BMI, prior to LVAD implantation. This must be done with care, considering the inherent risks of the supplementation strategy itself. For example, with the above-noted mechanisms leading to difficult alimentation in heart failure patients, a parenteral strategy is tempting. However, studies have shown an increased risk for device infection (particularly fungal infection) in patients with an LVAD receiving total parenteral nutrition (9).
Conclusions
Our two patients provide distinct challenges in assessing their peri-operative risk prior to LVAD implantation. Patient 1 appears to be at low risk for RV failure and bleeding [normal CVP and RVSWI, adequate renal function, low aspartate aminotransferase (AST), low MELD score] but is likely malnourished based on his low albumin, total protein, absolute lymphocyte count, and BMI. It would be reasonable to involve a nutrition specialist to consider supplementation strategies for this patient prior to implantation. Patient 2 is at high risk for both RV failure and bleeding. In this patient, hemodynamic optimization strategies (diuresis, inotropic support, afterload reduction, consideration of intra-aortic balloon pump placement) may mitigate her risk for both post-operative complications.
To this point, studies of patients prior to mechanical circulatory support have been largely observational or retrospective, and serve to provide a profile of patients at high risk. More prospective studies are required to define strategies to effectively optimize the moderate or high-risk patient prior to mechanical circulatory support.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail 2010;12:434-43. [PubMed]
- Genovese EA, Dew MA, Teuteberg JJ, et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg 2009;88:1162-70. [PubMed]
- John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008;86:1227-34; discussion 1234-5. [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [PubMed]
- Suarez J, Patel CB, Felker GM, et al. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail 2011;4:779-84. [PubMed]
- Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009;137:208-15. [PubMed]
- Matthews JC, Pagani FD, Haft JW, et al. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation 2010;121:214-20. [PubMed]
- Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant 2010;29:616-24. [PubMed]
- Maniar S, Kondareddy S, Topkara VK. Left ventricular assist device-related infections: past, present and future. Expert Rev Med Devices 2011;8:627-34. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Dang NC, Topkara VK, Kim BT, et al. Nutritional status in patients on left ventricular assist device support. J Thorac Cardiovasc Surg 2005;130:e3-4. [PubMed]
- Holdy K, Dembitsky W, Eaton LL, et al. Nutrition assessment and management of left ventricular assist device patients. J Heart Lung Transplant 2005;24:1690-6. [PubMed]





