VATS lobectomy lymph node management
Introduction
Despite 20 years of development and published reports of thousands of cases (1), major pulmonary resections by videoassisted thoracic surgery (VATS) techniques have only recently experienced the uptake which was observed previously in other fields of minimally invasive surgery. One of the commonly stated reasons was the inability to perform an adequate complete mediastinal lymph node dissection, thus rendering the technique oncologically inadequate. On one occasion, I witnessed a prominent thoracic surgeon state this as “fact” during a major international lung cancer conference. Even before definitive evidence to the contrary became available, I felt this was a preposterous stance on several levels.
Firstly, population-based studies have shown that the adequacy of lymph node sampling, let alone complete mediastinal lymph node dissection, is extremely variable, and generally variably performed in open lobectomy cases (2,3). Secondly, this stance assumed that a surgeon who was capable of carefully dissecting out the unforgiving pulmonary artery, finding and dividing all of the correct bronchovascular structures for the diseased lobe, and completing a difficult fissure, was somehow incapable of removing well defined anatomic areas of lymph node-bearing fat. Thirdly, albeit more recently, it has been shown in the American College of Surgeons Oncology Group (ACOSOG) Z0030 study, that if a single node at each lymph node station is negative on frozen section (or previous mediastinoscopy), then a complete node dissection is not required for an oncologically optimal lung cancer procedure (4).
Lymph node management at VATS lobectomy is therefore no different than it should be at lobectomy by thoracotomy. The first part of this perspective will therefore deal with the definitions of lymph node management and the evidence-based indications for sampling or complete mediastinal lymph node dissection regardless of surgical access. The remainder will deal with the practicalities of the VATS approach.
Definitions
Nodal Map in this perspective refers to the lymph node stations as charted by Rusch et al. for the International Association for the Study of Lung Cancer’s 7th edition TNM staging system (5).
Complete mediastinal lymph node dissection (CMLND) is also known by various names such as systematic lymph node dissection, complete or radical mediastinal lymphadenectomy, and extended mediastinal lymph node dissection. There is no consensus on the ultimate radicality of these procedures as it can include bilateral or N3 level node dissection for some surgeons. I define this as a similar dissection as was demanded in the ACOSOG Z0030 trial protocol (4), with the exception that I do not routinely dissect station 2 L, and only dissect station 4 L if there is an intra-operative finding of a microscopically involved station 5 or 7 node.
Systematic node sampling (SNS) refers to either taking a single node at each numbered station as in the control arm of ACOSOG Z0030, or 2 nodes from each field or station with at least 3 fields dissected always including station 7 (European Society of Thoracic Surgeons recommendation) (6).
More minimal forms of sampling are characterized a random biopsy or by the surgeon’s impression that particular nodes may be involved (the chance node), or use of sentinel node identification by Geiger counter and frozen section (the decision node) (7). Throughout this perspective I will be defining SNS as specified in the ACOSOG Z0030 trial protocol.
Indication for complete mediastinal lymph node dissection
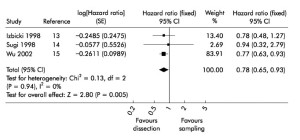
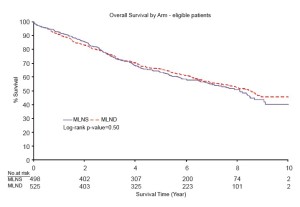
CMLND has been the subject of controversy ever since lobectomies were accepted as standard therapy for nonsmall cell lung cancer (NSCLC). In 2006, a systematic review and meta analysis was published which suggested a moderate survival benefit for complete mediastinal lymph node dissection (Figure 1) (8). The hazard ratio was 0.78, which is similar to the benefit of adjuvant chemotherapy in Stage II-IIIA NSCLC. The ACOSOG Z0030 trial accrued over 1,100 patients from 1999-2004 and included patients from USA, Canada and my own institution in Melbourne, Australia (4). Patients in this trial had SNS, then frozen section analysis. If they were found to be node-negative for the mediastinal and hilar nodes, they were randomized to CMLND or no further lymph node treatment. This trial showed no survival benefit of CMLND after SNS in highly selected early stage NSCLC (Figure 2).
In light of this partially contradictory evidence, reanalysis of all CMLND randomized trials now highlighted the differences of this trial and Sugi’s trial of small peripheral stage I adenocarcinoma (9) compared to the higher stage and more histologically diverse trials of Wu and Izbicki (10,11). The upshot of the evidence is now that CMLND remains standard of care for stage II-IIIA NSCLC (or unstaged NSCLC), but is not necessary for pathologically proven Stage I NSCLC (by pre-operative or intra-operative sampling by SNS).
Thoracic surgeons, regardless of whether performing VATS or thoracotomy, have to decide on their own protocol for lymph node management in the light of the above evidence and the realities of surgical practice. In my opinion, the cost and time wasted for SNS and frozen section analysis has outweighed the small additional time it takes for a complete mediastinal lymph node dissection. Given that the ACOSOG Z30 trial showed no clinically important difference in morbidity and the same survival, I routinely perform CMLND to ensure I get optimal staging (for adjuvant chemotherapy decisions) and any possible therapeutic advantage (in unexpected N1 or N2 disease).
VATS lobectomy lymph node management
Cheng et al. (12) and several other groups (13-16) have shown that the lymph node yield is similar by VATS or thoracotomy. In particular, in a prospective trial setting, a sub-analysis of the abovementioned ACOSOG Z0030 study showed no difference between VATS and thoracotomy for node dissection (15), and the only long term survival evidence showed no difference between a VATS approach and a thoracotomy approach (17). The difference between VATS and thoracotomy management therefore lies only in the tips and techniques required to obtain the appropriate sampling or complete dissection.
The approaches to VATS lobectomy itself vary; therefore I must set the scene for this overall perspective. Our routine VATS lobectomy consists of two standard thoracoscopic port sites, usually in the 7th and 8th interspaces, and a utility incision (non-rib-spreading mini-thoracotomy incision) varying from 3-8 cm depending on the size of the tumour. The hilum is usually dissected from anterior to posterior (upper and middle lobes) or from inferior to superior (lower lobes).
Technique overview
Using our approach, it is advantageous to dissect out many of the nodes required for sampling or complete dissection prior to dividing individual hilar structures. For example, if the inferior pulmonary ligament needs dividing to access the inferior pulmonary vein, station 9 is taken radically, which leaves most of this vein skeletonised. We would not simply divide the vein at this stage, but instead remove station 8 nodes posterior and superior to the inferior pulmonary vein and station 11 nodes between the vein and the lower lobe bronchus. This makes for a much simpler division of the vein, and subsequently the dissection of the bronchus. Similarly, we would advocate removal of any further station 10 L and 11 L nodes on the left side prior to division of the bronchus, as this then makes later dissection of the bronchus from the pulmonary artery much easier and safer.
Once the lobectomy specimen is removed there is more room to operate, oozing and back-bleeding is no longer a hindrance, and visualization of the required lymph node zones is simpler. The best approach is now to perform a systematic, if not complete, dissection of the anatomic lymph node stations that have not already been sampled or dissected.
On the right, I begin with the superior mediastinal node stations 2R, 4R and 10R. These can be dissected as a single bloc, or separately dissected from the three zones. If the specimen is removed as a single bloc, it needs to be (somewhat arbitrarily) divided into the three zones before submission to the pathologist, so that accurate staging is achieved. The azygos vein can be divided to facilitate a more radical dissection of the upper mediastinum, but this is not necessary. In most cases I remove station 2R and 4R, together with the azygos vein looped and retracted inferiorly, then remove station 10R with the azygos vein reflected superiorly.
Station 7 and 8 can usually be removed as a single bloc, and then separated at the point where the most prominent vagal branch to the lung was previously divided. This
anatomical landmark is my own rule, as there is actually no strict definition of where station 7 ends and station 8 begins.
Finally station 9 is taken, although this is probably the least likely to be involved if the resection specimen is not the lower lobe.On the left, I dissect station 5 and 6 as a single bloc if possible, clearing all of the tissue between vagus and phrenic nerves, and taking care not to damage the recurrent laryngeal nerve. Unless performing a radical dissection for a frozen section-proven positive node at station 5 or 7, I do not routinely dissect station 4 L due to the increasing risks of recurrent laryngeal nerve injury and the decreasing benefits of such radical lymph node excision. When proceeding to dissect station 4 L, I divide the ligamentum arteriosum to obtain better access. Alternatively, if the status of station must be known, it can readily be biopsied pre-operatively by mediastinoscopy or endobronchial ultrasound guided fine needle aspiration.
Advanced access and retraction techniques
Port-site seeding is a rare but recognized risk, which does not appear to be reduced by use of a specimen retrieval bag (1). However, we routinely use wound protectors that completely exclude the chest wall access tissues from the operation, while providing gentle radial retraction. I use a rigid small Alexis® retractor (Applied Medical, CA, USA) for the utility incision, and an extra-extra-small Alexis® retractor with removal tether for the posterior port site. This allows simple removal of the lymph node specimens without the need for multiple specimen retrieval bags.
CMLND is best performed from the highest accessible interspace (preferably the 4th). Therefore, on deciding upon the correct interspace to place the utility incision, I would advise a higher space if there is any doubt. Often a lower lobectomy is more easily performed an interspace below that of an upper lobectomy, and this does steepen the angle of approach of instruments to the superior mediastinal node stations. If this proves to be a significant obstacle, a fourth port can be placed in the auscultatory triangle for this part of the procedure.
We have found that the best instrument for grasping lymph nodes or associated fat is an angled sponge-holder. We then use an endoscopic version of a Cobb periosteal elevator (or a standard Cobb elevator if it reaches) to dissect away the tissues defining the lymph node package and for thinning out lymphatic vascular pedicles.
Haemostasis must be meticulous, as bleeding or chyle leak from the bed of station 7 or 4R can result in an unplanned return to the operating theatre. I use endoscopic clips liberally, although an ultrasonic or other haemostatic energy source could be employed as an alternative. In particular, there is commonly a small vein draining the station 4R package directly into the superior vena cava. This should be sought out and clipped early, and doing so will facilitate a better dissection.
Considerable anterior retraction of the lung is required for access to station 7. This can be a frustrating endeavour, depending on the lobe that has been removed. An angled sponge-holder can be placed through the utility incision to grasp the posterior aspect of the lower lobe and/or upper lobe and then used to pull the lung forward. This can then be left in the base of the utility incision (and even clipped to a drape to maintain retraction) while dissection carries on beside its shaft.
Acknowledgements
Conclusions There is no reason that a lobectomy by VATS should have any less optimal SNS or CMLND than by thoracotomy. All anatomical sites are accessible to standard VATS access. Surgeons who can perform VATS lobectomy already have the requisite surgical skills (although may need specific training). Multiple studies have confirmed their oncological equivalence based on lymph node yields and survival. The emergence of this “dilemma” of lymph node management by VATS at this time, is however fortuitous. It allows the dissemination of latest evidence related to lymph node management (and its importance for adjuvant chemotherapy selection) to both the new generation and the older generation of thoracic surgeons, regardless of whether they choose a VATS approach to lobectomy.Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6.
- Ellis MC, Diggs BS, Vetto JT, et al. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg 2011;92:1958- 63; discussion 1963-4.
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004; discussion 1005-6.
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70.
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92.
- Amer K. Video-Assisted Thoracic Surgery (VATS) Systematic Mediastinal Nodal Dissection. Topics in Thoracic Surgery 2012, P247-72.
- Wright G, Manser RL, Byrnes G, et al. Surgery for nonsmall cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597-603.
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-smallcell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5.
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6.
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44.
- Cheng A, Lim WM, Wainer Z, et al. Comparing VATS and open mediastinal lymph node dissection according to the proposed nodal zones of the IASLC staging committee. ANZ J Surg 2009;79:A80.
- Shigemura N, Akashi A, Nakagiri T, et al. Complete versus assisted thoracoscopic approach: a prospective randomized trial comparing a variety of video-assisted thoracoscopic lobectomy techniques. Surg Endosc 2004;18:1492-7.
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736.
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3.
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7.
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1.





