The Jarvik-2000 ventricular assist device implantation: how we do it
Introduction
The Jarvik-2000 (Jarvik Heart, Inc.; New York, NY, USA) is a valveless electrically powered non-pulsatile axial-flow left ventricular assist device (LVAD) that has been developed and refined over the last 25 years. It consists of a miniaturized intraventricular blood pump that lacks a real inflow conduit. The device is placed on the cardiac apex and creates a blood flow that can be directed either towards the ascending or descending aorta through its outflow conduit. Nowadays, it can be employed as a bridge to transplant or for permanent use (destination therapy) in case of untreatable end-stage heart failure (1-3).
The device
The Jarvik-2000 is 2.5 cm wide, 5.5 cm long and weighs 85 grams. The pump has one moving part, an impeller that is a neodymium-iron-boron magnet and which is housed inside a welded titanium shell. It is supported by ceramic bearings and spins blood to generate an average flow rate of 5 L/min (up to 7 L/min) with a rotation speed of 8,000-12,000 rpm (4). The device is connected to an external controller via a tunneled driveline that delivers power to the impeller. This controller permits manual adjustments of the pump speed and also visualizes the battery charge level.
Implantation strategies
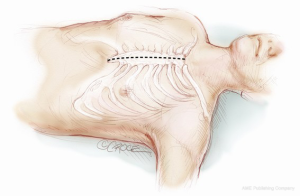
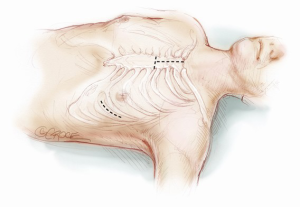
Many different surgical approaches have been described for the Jarvik implantation. The first surgical techniques required total cardiopulmonary bypass (CPB) support, whether the case of a median sternotomy access (Figure 1) or a single left lateral thoracotomy approach (Figure 2) (5). Newer and less invasive strategies make an off-pump implantation feasible and reproducible, performed either through a single left thoracotomy (Figure 2) or a combined left antero-lateral mini-thoracotomy and upper minimal inverted T or J sternotomy (Figure 3) or a double mini-thoracotomy (left antero-lateral thoracotomy plus upper right thoracotomy) (6,7). Only if necessary, peripherally inserted ECMO (ExtraCorporeal Membrane Oxygenation) can be used for hemodynamic support during the procedure instead of CPB institution. In both cases, it is necessary to create a subcutaneous tunnel to bring the power cable either to the abdominal region or the retro-auricular region (Figure 2); here, the external cable can be connected to the controller (8-10). The masthoid region is usually chosen to house the skull-pedestal for the power cable skin exit, as it is associated with minimal infective risk.
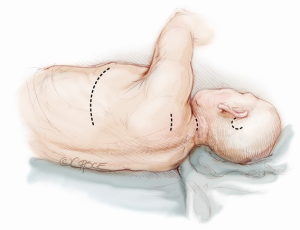
In the following paragraphs, we will describe how to perform the different steps of the Jarvik-2000 implantation using a left lateral thoracotomy or the combination of the mini-sternotomy plus the left antero-lateral thoracotomy. For the time being, the antero-lateral thoracotomy accompanied by the mini-sternotomy without CPB support has been used in eight cases out of 31 Jarvik device implantations at our institution; 15 patients were operated on through a left lateral thoracotomy, two cases through a median sternotomy and six patients were more recently approached through a double thoracotomy.
Operative technique
Anesthesia
The Jarvik implantation is performed under general anesthesia; induction is commonly achieved with sodium thiopental (midazolam and/or ketamine could be used alternatively), fentanyl and rocuronium, meanwhile maintaining drugs are generally propofol and sufentanil. Considering hemodynamic lability of the patients who require VAD implantation, an anesthetic conduction that aims to an early awakening and extubation with an adequate pain control can improve the patient management and lead to a good immediate postoperative course with a faster recovery. For this purpose, mild general anesthesia preparation with paravertebral analgesia has been used with clinical good results.
Positioning and preparation
The operative room is set up as for a conventional cardiac surgery, with a stand-by ECMO or CPB. Under general anesthesia (as mentioned before), the patient is intubated with a single lumen endotracheal tube; both central and peripheral venous lines are established (large volume infusion catheters are needed in case of significant hemorrhage) as well as an arterial line, followed by the insertion of a transesophageal echocardiography (TEE) probe. The patient position depends on the surgical approach: if the outflow conduit is connected to the ascending aorta, the combination of upper sternotomy and left lateral thoracotomy is necessary and the patient will be supine on the operating table (a roll is placed under the left chest in order to turn the patient slightly rightward—about 30 degrees). When the descending aorta is chosen as the outflow conduit connection site, a left thoracotomy will be performed and the patient will be positioned in the lateral decubitus position. In both cases, a sterile preparation of the temporo-occipital area, the neck, the thorax and the groin is mandatory; above all, free access of the left femoral vessels for percutaneous cannulation has to be available for all the procedures in case of hemodynamic instability and the need for ECMO support.
Surgical access 1: upper mini-sternotomy and left mini-thoracotomy
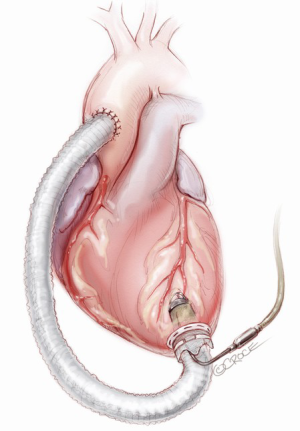
When the Jarvik-2000 is planned to convey blood towards the ascending aorta (Figure 4), the surgeon enters the mediastinum performing an anterior left mini-thoracotomy through the fifth intercostal space, in order to access the apex and an inverted T or J upper mini-sternotomy through the second/third intercostal space to access the ascending aorta. A tissue retractor can facilitate the surgical exposition of the apex, while a sternal retractor is used in the second access point.
Surgical access 2: left thoracotomy
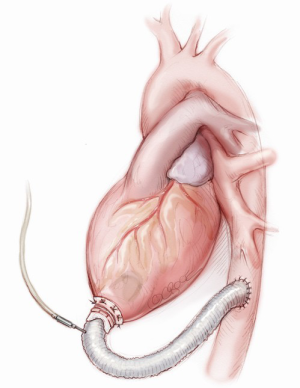
The choice to connect the outflow graft of the pump to the thoracic descending aorta (Figure 5) requires a single extended left thoracotomy. Adequate space to control the left postero-lateral aspect of the mediastinum is necessary and the incision is generally made through the sixth intercostal space from the emiclavear line to the left subscapular area, without sparing the serratus anterior muscle.
Retro-auricular site preparation
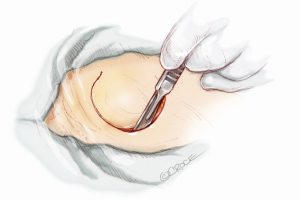
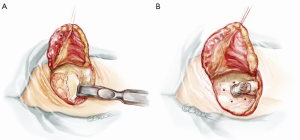
Before heparinization and starting the maneuvers on the heart and aorta, the skin exit for the power cable of the pump is created. A “C-shape” skin incision in the retro auricular region is performed in order to prepare a large-based flap slightly above the ear (Figure 6). The subcutaneous tissue is dissected and periosteum is slit (Figure 7A). Six holes are drilled in the bone with a specific 6 mm long drill (Figure 7B) and the pedestal is temporarily implanted to punch out the skin flap and identify the final exit for the external power connection.
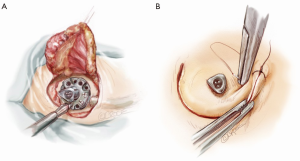
When a left lateral thoracotomy is performed, two other skin incisions in the postero-lateral side of the neck and shoulder are made to create a subcutaneous tunnel which extends from the upper chest to the mastoid region (the lower skin incision is just above the superior medial scapular angle) (Figure 2). When the Jarvik is moved into the field, the power-cable with the three-pin connector is guided through this tunnel and connected to the pedestal. This action is achieved by inserting the driveline within the end of a thoracic drain tube which is brought out through the second intercostal space (between the medial border of the scapula and the spine) to the cranial site; tapes assist in pulling the tube across the tunnel exits. The three-pin connector is then inserted into the titanium pedestal, which is eventually fixed with self-tapping screws (Figure 8A). More than one lateral cervical incision is useful in allowing more comfortable driveline positioning preserving neck motion without strain of the cable after the surgery. Once the pedestal is secured on the bone, the skin flap is repositioned, covering the skull implant but leaving the appropriate part of connector free for coupling with the external power support (Figure 8B). Testing of the pump concludes this phase.
Apical setting
The pericardium is horizontally opened anterior to the phrenic nerve and the apex is inspected; the appropriate site of the pump implantation is slightly anterior and 1-2 cm lateral to the descending coronary artery. The surgeon has to make sure that the ventriculotomy will be directed at the mitral valve, always keeping a parallel direction to the septum. A finger is pushed on the apex to confirm the insertion site for the Jarvik-2000 by using the TEE, also checking for parietal thrombi. Afterwards, the inflow sewing ring is sutured and tied to the myocardium using 10-12 interrupted pledgeted, double-armed 3-0 polypropylene sutures (Figure 9). Suture penetration is deep enough to ensure the sewing ring stabilization, but the bites should not span the full thickness of the muscle, especially when the myocardium appears thin and weak due to dilation or previous ischemia.
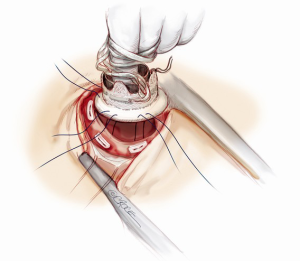
Device implantation with outflow graft in the descending aorta
The distal graft anastomosis is made first: the thoracic descending aorta is isolated and a side-biting clamp is placed on it, right adjacent to the inferior left pulmonary vein, avoiding dilated areas or atheromatous plaques. Here, the clamped graft is sewn with a running 4-0 polypropylene suture. Two Teflon strips are usually employed as structural reinforcement of the anastomosis and to ensure hemostasis.
After placing the patient in Trendelenburg position, a transmural cruciate incision is performed inside the sewing ring. A coring knife is then inserted to excise a ring of muscle. In a continuous motion, the knife is removed and the pump is quickly pushed into the ventriculotomy, trying to lose as little blood as possible (this maneuver is made easy by the small size of the pump). As the pump is placed in the apex, the internal connecting umbilical strings attached to the sewing ring are tied; the pump is then switched on at a low speed to start the de-airing process and the device is definitely secured in the retaining cuff closing the external umbilical strings. The system de-airing is then completed with a medium gauge needle and the left cardiac cavity is evaluated by TEE for residual bubbles and for the correct intraventricular device position confirmation.
Device implantation with outflow graft in the ascending aorta
After the inverted T or J upper mini-sternotomy is performed, the ascending aorta is isolated. The pump is brought up to the field, and the outflow conduit is tunneled underneath the pericardium from the left thoracotomy to reach the partial sternotomy (Figure 10). The apical setting and implantation of the pump is practically unchanged from the previous description. De-airing is performed by activating the pump at low speed, allowing blood to fill the outflow conduit. Once the surgeon has secured the pump into the apex, the outflow graft conduit is gently stretched; the right length is assessed and the conduit is cut. A partial occlusion clamp is placed on the anterior-lateral aspect of the ascending aorta, a longitudinal arteriotomy is performed and the graft is sewn in place using running 4-0 polypropylene sutures. Completion of air removal is accomplished by using a needle inserted into the outflow graft before opening the side-biting clamp. After TEE inspection of the heart chambers, the Jarvik-2000 is left free to convey blood to the aorta.
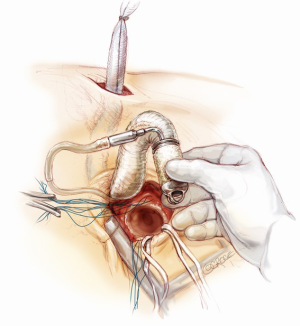
Conclusions of the procedure
The device speed is titrated and gradually increased following the hemodynamic condition, providing an adequate cardiac index (>2.2 L/min/m2) and maintaining low filling pressure of the left ventricle. Inotropic and vasopressor drugs are used to support the right ventricle and adjust the peripheral vascular tone. Protamine sulfate is used to reverse the heparin, mediastinal pleural drainage tubes are placed and the chest is closed in layers. Scalp and skin incisions are securely closed.
Perioperative comments
We have presented our techniques of Jarvik-2000 LVAD implantation without using CPB or ECMO support. The favored site for power-connector is the retro-auricular region. What we have showed here is quite a similar technique to that used to implant an HeartWare or a HeartMate ventricular assist device, except in the details regarding the apical inflow part of the pump and the tunneling of the power cable outside the organism. Such a surgical procedure is safe and reproducible, provided that it is carefully planned and preoperative evaluation of other coexisting structural heart disease that can alter the pump functioning is undertaken. In particular, the presence of an atrial septal defect/patent foramen ovale (ASD/PFO), aortic insufficiency, mitral stenosis and tricuspid regurgitation must be corrected at the time of surgery.
The reason for correcting the above mentioned diseases are explained by the blood flow physiology related to the pump. Firstly, ASD/PFO can lead to a significant right-to-left shunt and generate severe hypoxemia; aortic insufficiency can reduce the forward flow creating a circular flow circuit that involves the pump, outflow graft, aorta and left ventricle; mitral stenosis can reduce the pump preload, thus reducing the device performance; tricuspid regurgitation can affect the right ventricle function and again, reduce the adequate assist device preload.
Right ventricular failure is one of the most important concerns when a LVAD is implanted and is associated with increased postoperative morbidity and mortality. It is therefore crucial to evaluate the right ventricle risk profile preoperatively and predict whether stronger support strategies will be needed in the operative setting (inotropes, ECMO or even a temporary right ventricle assist device).
Even with the best medical and surgical care, complications can occur following Jarvik implantation, as with any other type of cardiac support device. The most frequent complications are (I) bleeding, due to coagulation factor alteration and activation, platelet modifications and abnormal liver function related to refractory heart failure; (II) cerebral and peripheral thromboembolism, for the same reasons as changes in coagulation state; (III) infections, related to foreign materials and the skin exit sites of cables; (IV) hemolysis, due to red blood stress created by the device impeller; and (V) arrhythmias, related to electrical instability of the myocardium, apical scarring and suction physiology.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Park SJ, Kushwaha SS, McGregor CG. State-of-the-art implantable cardiac assist device therapy for heart failure: bridge to transplant and destination therapy. Clin Pharmacol Ther 2012;91:94-100. [PubMed]
- Mallidi HR, Anand J, Cohn WE. State of the art of mechanical circulatory support. Tex Heart Inst J 2014;41:115-20. [PubMed]
- Hirsch DJ, Cooper JR Jr. Cardiac failure and left ventricular assist devices. Anesthesiol Clin North America 2003;21:625-38. [PubMed]
- Jarvik R. Jarvik 2000 pump technology and miniaturization. Heart Fail Clin 2014;10:S27-38. [PubMed]
- Westaby S, Frazier OH, Pigott DW, et al. Implant technique for the Jarvik 2000 Heart. Ann Thorac Surg 2002;73:1337-40. [PubMed]
- Selzman CH, Sheridan BC. Off-pump insertion of continuous flow left ventricular assist devices. J Card Surg 2007;22:320-2. [PubMed]
- Gerosa G, Gallo M, Tarzia V, et al. Less invasive surgical and perfusion technique for implantation of the Jarvik 2000 left ventricular assist device. Ann Thorac Surg 2013;96:712-4. [PubMed]
- Siegenthaler MP, Martin J, Frazier OH, et al. Implantation of the permanent Jarvik-2000 left-ventricular-assist-device: surgical technique. Eur J Cardiothorac Surg 2002;21:546-8. [PubMed]
- Siegenthaler MP, Martin J, Frazier OH, et al. Implantation of the permanent Jarvik-2000 left-ventricular-assist-device: surgical technique. Eur J Cardiothorac Surg 2002;21:546-8. [PubMed]
- Hohlweg-Majert B, Gutwald R, Siegenthaler MP, et al. Implantation of the Jarvik 2000 left-ventricular-assist-device: role of the maxillofacial surgeon. Eur J Cardiothorac Surg 2005;28:337-9. [PubMed]