Mechanical circulatory support devices as destination therapy—current evidence
Abstract
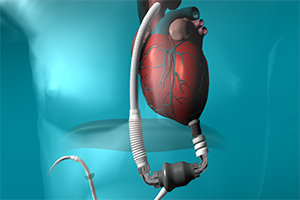
Advanced heart failure is an increasing problem worldwide. Nowadays, mechanical circulatory support devices (MSCD) are an established therapeutic option for terminal heart failure after exhaustion of medical and conventional surgical treatment, and are becoming a realistic alternative to heart transplantation (HTX). There are a number of different treatment options for these patients, such as bridge to transplantation (BTT), bridge to candidacy (BTC), bridge to recovery (BTR) and the destination therapy (DT) option. The latter option has become more frequent throughout the last years, due to a donor organ shortage and an increasing number of older patients with terminal heart failure who are not eligible for HTX. These factors have led to a rapidly increasing number of LVAD implantations as well as centers which perform these procedures. This has also been due to improved LVAD survival rates and quality of life following the introduction of smaller, intrapericardial and more durable continuous flow left ventricular devices. The most common complications for these patients are device-related problems, such as coagulation disorders, gastrointestinal bleeding, device related infection, pump thrombosis or cerebrovascular accidents. However, some questions still remain unanswered or under debate, such as the exact time-point for LVAD implantation. In addition, aspects such as better biocompatibility for LVADs remain a major challenge. This review will concentrate on DT for terminal heart failure and provide an overview of the current evidence for LVAD implantation in this patient group, with particular emphasis on indication and time-point of implantation, choice of LVADs, and long term outcomes and quality of life.
Cover