Medical management in type B aortic dissection
Introduction
The aim of treatment in aortic dissection is to limit propagation of the false lumen and its negative consequences on end-organ perfusion by reducing and stabilizing hemodynamic stress on the aortic wall (1-8). As the majority of these type B dissection patients are hypertensive, medical therapy is centered on the use of anti-hypertensive agents. Medical therapy also aims to maintain hemodynamic stability in the chronic phase to promote aortic stability and to prevent aortic expansion, which might cause possible rupture and/or recurrent dissection.
Medical management of aortic dissection is still based mainly on personal experience, expert opinion and historical observational studies as there is a paucity of randomized controlled studies (1-8). As a result, in the last decade, efforts have been made to better understand the medical management of the disease. These efforts have ranged from proposal of guidelines from European, American and Asian societies, to the analysis of the International Registry of Acute Aortic Dissection, which is presently the largest and most comprehensive global registry database for this disease (9).
Guideline-based medical management
European guidelines
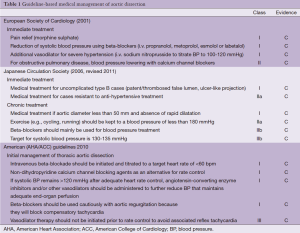
The European Society of Cardiology (ESC) was the first society to publish guidelines on aortic dissection in 2001 (2). In the initial assessment of the disease, immediate management of pain and blood pressure, with the target of lowering systolic blood pressure to 100-120 mmHg was recommended. For this, morphine sulfate is typically used for pain control and beta-blockers are most favored to reduce the force of left ventricular ejection (dP/dt), which will otherwise continue to weaken the arterial wall. Detailed recommendations were also presented on the use of beta-blockers, especially on intravenous use (e.g., loading and maximal dose) for agents such as propranolol, esmolol, metoprolol, atenolol, and labetalol. In patients who do not tolerate beta-blockers well, such as patients with bronchial asthma, bradycardia or signs of heart failure, esmolol was stated to be a reasonable choice to test the patients' reaction to beta-blockers given its short half-life compared to metoprolol. The guidelines note that there is no data supporting the use of calcium antagonists (e.g., verapamil, diltiazem, or nifedipine) but also suggest that these drugs may be necessary to reduce blood pressure, particularly in patients with bronchial asthma. In cases where beta-blockade alone is insufficient to control hypertension, vasodilators were recommended as an ideal additional agent to control blood pressure; however, they should always be combined with beta-blockers because they can increase the force of left ventricular ejection. While beta-blocking agents are usually adequate in patients with slightly elevated blood pressure, it was noted that combined use of beta-blockers with intravenous sodium nitroprusside might also be required for more severe hypertension. Finally, the guidelines recommended that lowering of systolic blood pressure needs to be modified if oliguria or neurological symptoms develop.
It must be noted however, that the ESC guidelines did not make recommendations on long-term management but focused specifically on management in the acute phase with the inference to continue this as definitive treatment as necessary. Furthermore, these guidelines did not make recommendations on heart rate as mentioned in the American guidelines. Given that other guidelines have become more recently available, these items may be discussed in an upcoming update in the near future.
Asian (Japanese) guidelines
The next available guidelines emerged from Japan in 2006 and were updated in 2011 (3). Medical management is classified according to acute (immediate) and chronic phases. Aims in the acute phase are to control blood pressure (100-120 mmHg), heart rate and pain. While this blood pressure target is generally accepted, there is a lack of robust evidence supporting this. Furthermore, it is thought that pain reflects the extension of the dissection, and alleviation of pain through blood pressure management will be beneficial.
In regards to specific anti-hypertensive agents, intravenous use of nicardipine, nitroglycerin and diltiazem in combination with beta-blockers was recommended. Intravenous use in the immediate phase is preferred given the ease of titration with transition to oral agents. It was noted that there is little evidence on oral agents. The use of beta-blockers was recommended to control heart rate to preferably less than 50 bpm in the immediate phase and to reduce dissection-related events in the chronic phase. Morphine or buprenorphine were also recommended to control persistent pain. After the immediate phase, blood pressure control should aim between 100-120 mmHg with some flexibility. In the chronic phase, it was emphasized that blood pressure control is important as favorable blood pressure control can reduce re-dissection by two-thirds. Only beta-blockers have evidence stating that they can reduce dissection-related events and inhibit aortic dilatation. There are reports stating that systolic blood pressure targets at 130 mmHg or less than 135/80 mmHg are appropriate, but again there is a lack of clear evidence. In cases with visceral ischemia, targets may need to be lowered. During rehabilitation, it was noted that systolic blood pressure preload should be kept below 130 mmHg and afterload to less than 150 mmHg.
Furthermore, uncomplicated type B dissections have a 30-day mortality rate of less than 10%, a rate which is comparable to surgical outcomes, and therefore medical treatment is appropriate in the acute phase. However, surgery should be considered for complicated cases, and that thoracic endovascular aortic repair (TEVAR) has shown promising results as a new and alternative option. Notably, patients resistant to anti-hypertensive therapy are not necessarily indicated for surgical management as they previously were, given that recent reports have suggested that elevated blood pressure is not necessarily a cause of increased risk for rupture. In the chronic phase (after two weeks), medical treatment should be continued for stable patients as they generally have a favorable prognosis.
American guidelines
The American Heart Association/American College of Cardiology (AHA/ACC) guidelines were published in 2010 and classify aortic dissection among the acute aortic syndromes. These guidelines note that 71% of patients that sustain type B aortic dissections have a systolic blood pressure greater than 150 mmHg at presentation. Initial management of thoracic aortic dissection was recommended to decrease aortic wall stress by controlling heart rate and blood pressure.
Initial medical stabilization using beta-blockers was recommended to control aortic wall stress that is affected by the velocity of ventricular contraction, the rate of ventricular contraction, and blood pressure parameters. Initial targets of heart rate less than 60 bpm and systolic blood pressure between 100-120 mmHg were recommended in order to maintain adequate end-organ perfusion. It should be noted that unlike the European guidelines, the American guidelines emphasize heart rate control. Intravenous propranolol, metoprolol, labetalol, or esmolol are suggested as excellent choices for initial treatment. In patients who are unable to tolerate beta-blockade, non-dihydropyridine calcium channel antagonists (verapamil, diltiazem) were suggested to offer acceptable, although less-established, alternatives. The use of beta-blockers, verapamil or diltiazem for rate control in patients with significant aortic regurgitation was noted to be potentially problematic due to deleterious effects on reflex tachycardia. In cases where vasodilators may be required to control blood pressure in addition to beta-blockade, intravenous sodium nitroprusside is the most established agent and offers the advantage of being rapidly titratable. It is important to consider, however, that vasodilator therapy without prior beta-blockade may cause reflex tachycardia and increased force of ventricular contraction leading to greater aortic wall stress and potentially cause false lumen propagation. Nicardipine, nitroglycerin and fenoldopam were also listed as being appropriate. Following initial stabilization with intravenous antihypertensives, most patients will require long-term antihypertensive treatment including the use of a beta-blocker plus additional classes of agents. It was noted that angiotensin-converting enzyme inhibitors or angiotensin receptor blockers may also retard aortic dilatation and that their use may be indicated.
Findings from the International Registry of Acute Aortic Dissection
The International Registry of Acute Aortic Dissection (IRAD) is the world’s largest multi-center registry-based study focused on aortic dissection to understand the clinical profiles, diagnosis, treatment and outcomes of the disease. Medical management of aortic dissection was addressed by analyzing the IRAD global registry database (579 type B cases). Data regarding medication prescription to patients with type B dissections at discharge was analyzed to investigate the association of medications and mortality. Initial univariate analysis showed that use of beta-blockers was associated with improved survival in all patients (P=0.03), and that use of calcium channel blockers was associated with improved survival in type B patients receiving medical management (P=0.03). Multivariate models further showed that use of calcium channel blockers was associated with improved survival in type B medically-treated patients (OR 0.55; 95% CI, 0.35-0.88, P=0.01). The findings of the IRAD analysis collectively demonstrated that while use of beta-blockers was associated with improved outcome in all patients with dissection, the use of calcium channel blockers was associated with improved survival selectively in type B dissections. Use of ACE inhibitors did not improve survival. Consequently, the IRAD analysis suggests the possibility of type-selective benefits of medications in acute aortic dissection.
Conclusions
Recent society guidelines and findings from global registry databases (e.g., IRAD) have made significant contributions to our approach to medical management of acute type B aortic dissection. Close examination of guidelines show that each emphasizes control of different parameters (see Table 1). Looking to the future, these guidelines will serve as a working model to shape our medical management of dissection and the question of preferred treatment should only be revisited after they have been thoroughly implemented. If a randomized controlled trial ever becomes possible for dissection given ethical concerns, a definitive answer on the optimal treatment of the condition may become clearer.
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [PubMed]
- Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011): digest version. Circ J 2013;77:789-828. [PubMed]
- Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation 2005;112:3802-13. [PubMed]
- Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55-66. [PubMed]
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 2003;108:628-35. [PubMed]
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part II: therapeutic management and follow-up. Circulation 2003;108:772-8. [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [PubMed]
- Suzuki T, Isselbacher EM, Nienaber CA, et al. Type-Selective Benefits of Medications in Treatment of Acute Aortic Dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol 2012;109:122-7. [PubMed]





