Possible graft-related complications in visceral debranching for hybrid B dissection repair
Introduction
The search for less invasive solutions for the treatment of thoracoabdominal aortic pathology and the progression of endograft technology has led to the development of hybrid techniques, which combine open surgery with endovascular procedures (1).
Advantages of hybrid repair (HR) include the avoidance of thoracotomy, phrenotomy and aortic cross clamping. Theoretically, this limits the risk of respiratory failure, and ischemia-reperfusion injury (2). However, new procedure-related complications have emerged (3). In this paper, we retrospectively analyzed the clinical outcomes, patency and hemodynamic alterations of the renovisceral debranching grafts in our series of thoracoabdominal aortic aneurysm (TAAA) HR in order to determine the role of aortic dissection, graft routing and angulation as risk factors for procedure-specific complications.
Methods
Indications and operative technique
From 2001, we identified 55 high-risk patients (42 males, median age 71.9 years with an interquartile range, 23 to 83 years) with 44 atherosclerotic and 11 dissecting TAAAs who underwent HR with visceral aortic debranching and endovascular exclusion of the aneurysm. Inclusion criteria for hybrid TAAA repair were high-risk patients defined by American Society of Anaesthesiologists (ASA) class 3 or 4, associated with forced expiratory volume in 1 second <50%, or cardiac ejection fraction <40%. One patient who did not fulfill these clinical criteria was selected for HR due to retroperitoneal fibrosis and “frozen chest”. Forty-two (76.4%) patients had undergone previous aortic surgery. The mean maximal TAAA diameter on the short axis was 68 mm (range, 64-91 mm). One patient underwent emergency treatment for TAAA rupture. Anatomic inclusion criteria for HR included a minimum proximal aortic neck length of 20 mm, the potential efficacy of aortic visceral debranching in lengthening the distal aortic landing zone to greater than 15 mm, and an aortic neck diameter allowing endograft oversizing by 15% to 20% with the absence of circumferential thrombus or calcifications.
Thirty-nine simultaneous (70.9%) and 16 staged procedures (29.1%) were performed.
Visceral rerouting
One hundred and fifty-nine visceral bypasses were performed (156 retrograde; three anterograde). A two-vessel revascularization was completed in 25 cases (45.5%), a three-vessel revascularization in 11 cases (20.0%) and a four-vessel revascularization in 19 cases (34.5%). Fifty-one bypasses to the celiac trunk (CT), 54 to the superior mesenteric artery (SMA), 23 to the left renal artery (LRA) and 31 to the right renal artery (RRA) were performed. In thoracoabdominal aortic aneurysm dissection (TAAD) patients, dissection involved 9/44 (20.5%) renovisceral vessels. The estimated diameters of visceral bypasses were 6 mm in 123 cases and 8 mm in 36 cases, with 154 Dacron and five expanded-polytetrafluoroethylene grafts employed. Customized Y-graft and single bypass were the preferred configurations, although reversed bifurcated or trifurcated grafts and single bypasses with sequential graft technique were also used. Retropancreatic graft routing for CT revascularization was initially our preferred approach and was utilized in 19 cases, and the current technique involves an antepancreatic approach with end-to-side anastomosis on the common hepatic artery. End-to-end anastomosis was usually performed for the SMA and renal arteries.
One patient undergoing long-term dialysis had intentional coverage of both renal arteries (RAA), and six patients with a solitary functioning kidney had intentional coverage of the contralateral RA without revascularization. One patient had an aberrant common hepatic artery originating from the SMA, with a small anomalous CT, which was intentionally covered without revascularization. One patient had a gastro-hepatic trunk and the splenic artery originating independently from the aorta; the splenic artery was intentionally covered. In one patient with previous left nephrectomy and CT occlusion, only the right RA and the SMA were grafted. Another patient with previous open type IV TAAA conventional repair by aortic grafting with bevelled proximal anastomosis developed a “frozen chest”, severe retroperitoneal fibrosis and a type III TAAA, in addition to a non-functional left kidney. Antegrade revascularization of CT, SMA and right RA was performed through median sterno-laparotomy and by means of a trifurcated graft, attached to the ascending aorta and routed through the diaphragm.
In all cases, the grafted vessels were ligated proximally to prevent type II endoleak. The grafts were then covered with retroperitoneum or omental flap whenever possible.
Completion angiography was performed after debranching or before endograft deployment in staged procedures in 36 (65.5%) patients, in order to assess the patency of visceral bypasses and to treat potential technical defects.
Patency of 19 (11.9%) visceral grafts in 10 patients was intra-operatively assisted. One RA dissection, 13 RA stenoses or occlusions, and five angulations with associated stenosis of the anastomosis of the SMA were detected during intra-operative angiography, and immediately corrected through deployment of a self-expanding stent (Figure 1). An initial dissection with mild stenosis of the right iliac artery, which represented the donor vessel for visceral rerouting, was also stented intra-operatively distal to the proximal anastomosis of a “Y” graft, due to a reduced femoral pulse following unclamping.

Four target vessel revascularization (LRA in three patients and CT in one patients) failed because of technical issues. The presence of dissecting pathology did not have a significant impact on the making of the bypasses.
Inflow site
The choice of the inflow site for retrograde bypasses was based on the extent of the TAAA, the presence of prior abdominal aortic surgery, and the quality of the native aorta and iliac arteries.
Patients with native aorto-iliac vessels (29 cases)
Among the 29 patients without prior abdominal aortic grafting, the native aorta represented the donor vessel in six cases, while the common iliac artery was used as the inflow vessel in 17 cases. In the other six patients, the infra-renal aorta was replaced for synchronous AAA during hybrid TAAA repair (tube graft in four patients; aorto-bi-iliac bypass in two patients). The visceral bypasses were pre-sewn or anastomosed to the distal part of the tube graft, or to the main body/iliac branches in patients with aorto-iliac bypass. They were then routed to the target vessels.
Patients with pre-existing abdominal aortic graft (26 cases)
In 20 patients with prior AAA or TAAA aortic grafts repair, renovisceral bypasses were anastomosed to the graft. In five cases with abdominal aortic graft, common iliac arteries were the vessels’ inflow site, due to good arterial quality and favorable anatomy. One patient with pre-existing abdominal aortic graft had retroperitoneal fibrosis and “frozen chest”. In this patient, three antegrade bypasses from the ascending aorta were performed.
Follow-up
Patients were evaluated with post-procedure contrast CT scans at scheduled follow-up intervals of one, six and 12 months, and yearly thereafter. Clinical follow-up was also performed at regular 6-month intervals. Angles between grafts and target vessels were assessed.
Statistical analysis
Visceral graft-related complications were defined as graft occlusion, graft infection and pancreatitis. Univariate analysis was performed to investigate the association of possible risk factors with the development of graft-related complications using a linear regression model for continuous variables, and the analysis of variance (ANOVA) for categorical variables. Variables that presented an association (P≤0.05) at univariate analysis were entered in a stepwise multiple regression model as possible predictors of visceral complications.
The null hypothesis for statistical tests was rejected at P<0.05. All analyses were run using IBM SPSS Statistics 18 software (SPSS Inc, Chicago, IL, USA).
Results
Perioperative results
No intraoperative deaths occurred. Thirty-day mortality was 12.7% (seven patients), including multiple organ failure in two, myocardial infarction in two, coagulopathy in one, pancreatitis in one and bowel infarction due to CT and SMA grafts occlusion in one patient.
Overall major perioperative morbidity was observed in 16 (29.1%) patients. Potential graft-related complications included four cases of pancreatitis (7.3%), with associated abdominal fluid collection requiring percutaneous drainage, and five cases of peri-operative renal failure (9.1%) resolved without dialysis.
Out of the five cases of pancreatitis, four had retropancreatic routing of the graft to the CT. No cases of renal failure were associated with graft occlusion.
Mid-term results
At a mean follow-up of 36.1±19 months, three patients had sudden deaths which were potentially related to aortic ruptures, but no post-mortem examinations were performed.
The global rate of visceral graft occlusion was 9.4% (15/159). Actuarial renovisceral grafts primary patency at 12, 24, and 36 months was 96.3%, 92.6%, and 90.2%, respectively. The SMA graft was occluded in four patients, leading to bowel infarction and death in two. Renal graft occlusion causing kidney loss was observed in seven cases. Four asymptomatic CT graft occlusions were recorded.
Graft occlusion rates were 7.8% (4/51) for the CT, 7.4% (4/54) for the SMA, 13.0% (3/23) for the LRA and 12.9% (4/31) for the RRA.
At 6-month follow-up, two high grade stenoses of the SMA anastomosis causing abdominal angina and significant weight loss were detected, and successfully treated with stenting. A severe anastomotic stenosis to the LRA was detected at one-year follow-up and treated with stenting as well.
One case of pancreatitis, one case of renal failure with no evidence of graft occlusion or stenosis and one case of debranching grafts infection managed with surgical drainage and omentoplasty (Figure 2) were also observed.
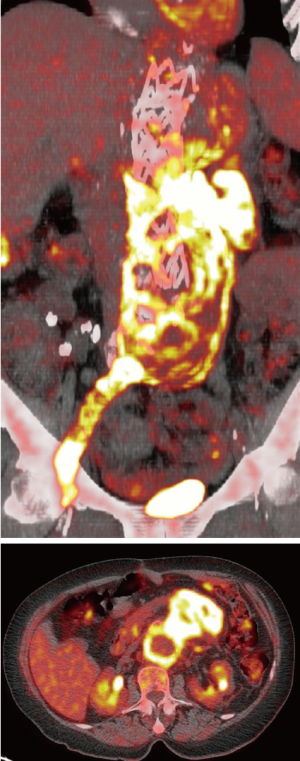
Eleven non-procedure-related deaths (three myocardial infarctions, two cancers, one cerebral aneurysm rupture, one head trauma and four unknown) were recorded. Six patients were lost to follow-up.
As measured at CT scan, the mean angles between graft and common hepatic artery, graft and SMA, and graft and RAA, were 92±3°, 56±2° and 98±2°, respectively. No significant angle modification was observed in CT and RAA graft occlusion or stenosis. An angle <40° between graft and SMA was associated with significant graft flow alterations (VPS >250 cm/s) at color flow Duplex (CFD), and was observed in 10 cases (18.5%).
Aortic dissection did not have a significant impact on visceral graft-related complications. Using univariate analysis, retropancreatic routing of graft to CT and angle between graft and SMA <40° were significant variables (P=0.007 and P=0.001 respectively). Using multivariate analysis, retropancreatic routing to CT was the only independent predictor for visceral complications (P=0.006) (Table 1).
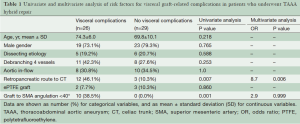
Full table
Discussion
Although open surgical repair of TAAA has evolved significantly over the last decades (4,5), this procedure still associated with significant morbidity and mortality, particularly in high-risk patients (6-8). Hybrid TAAA repair has emerged as a less invasive alternative and may currently represent a “bridge” solution until larger series and reproducible results from evolving experience in total endovascular TAAA repair become available (9,10).
Along with advantages offered by hybrid techniques (no thoracotomy, phrenotomy, extracorporeal circulation and aortic cross clamping), we observed the occurrence of new threatening specific complications, such as bowel infarction, pancreatitis and renal artery thrombosis.
Visceral bypass outcomes
Safe routing and long-term patency of visceral bypasses represent major concerns. Devastating complications such as pancreatitis and organ ischemia due to visceral graft occlusion must be carefully assessed.
In our series, we found that retropancreatic routing of the graft to CT is an independent risk factor for visceral complications. In fact, the pancreatic complications observed occurred during the first part of our experience, when retropancreatic routing was the preferred approach. When we switched to the antepancreatic routing, only one case of pancreatitis was observed. This may be due to the more traumatic manipulation of the pancreas during creation of the retropancreatic tunnel.
Patency rates of SMA grafting for atherosclerotic disease have been widely described (11), however, less is known about debranching in hybrid procedures. The SMA graft represented a major concern in our experience; stenting of the anastomosis was needed in seven cases and five grafts occluded leading to death in three patients. A graft angulation <40° was found to be associated with significant flow alterations and graft-related complications. Additionally, we observed no restenosis after stenting of the anastomosis. This fact can be explained by the stiffness of the stent, which may open the curve and reduce the angle between graft and SMA. For this reason, visceral grafts should be carefully tailored to the correct length. In fact, when the abdominal organs are repositioned, long grafts will easily kink and eventually occlude, while short bypasses may create excessive tension on the anastomoses and traction on the visceral vessels. In order to avoid these configurations, we made the visceral bypasses in the shape of a “lazy C” wherever possible; alternative graft configurations may be explored (Figure 3).
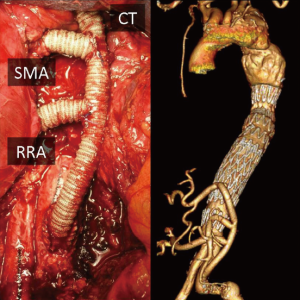
Baseline angiography of the visceral bypasses was performed in both single and staged procedures, with special care in checking for flow-limiting technical defects such as stenosis, visceral vessel dissection, and a kinked anastomosis or angulated or twisted grafts after bowel repositioning. In these cases, prompt correction with a self-expanding stent has been consistently safe and effective in our series.
The risk of late stenosis of visceral bypasses needs to be carefully assessed. Because of the risk of enteric erosion or fistulization of visceral grafts in their extra-anatomic route, adjunctive endovascular procedures are recommended in case of significant stenosis or angulation.
Interestingly, new sutureless telescoping anastomotic techniques using self-expanding endografts have been proposed to minimize vessel dissection and ischemia during renovisceral vascularization in hybrid TAAA repair (12).
In addition, techniques of partial visceral debranching combined with the use of covered periscope and chimney grafts have been shown to be feasible (13,14) and may represent an option in the treatment of complex thoracoabdominal aortic pathology.
Conclusions
Specific visceral graft-related complications are not uncommon in our series and are often associated with clinical consequences. Operative techniques play a role in the development of such complications. As a result, careful graft routing and alternative technical strategies to avoid excessive angulation of retrograde grafts should be carefully considered. Intraoperative angiography and strict follow-up are mandatory to monitor visceral bypasses and facilitate patency when needed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yamaguchi D, Jordan WD Jr. Hybrid thoracoabdominal aortic aneurysm repair: current perspectives. Semin Vasc Surg 2012;25:203-7. [PubMed]
- Hughes GC, Andersen ND, Hanna JM, et al. Thoracoabdominal aortic aneurysm: hybrid repair outcomes. Ann Cardiothorac Surg 2012;1:311-9. [PubMed]
- Tshomba Y, Melissano G, Logaldo D, et al. Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms. Ann Cardiothorac Surg 2012;1:293-303. [PubMed]
- Coselli JS. The use of left heart bypass in the repair of thoracoabdominal aortic aneurysms: current techniques and results. Semin Thorac Cardiovasc Surg 2003;15:326-32. [PubMed]
- Schepens M, Dossche K, Morshuis W, et al. Introduction of adjuncts and their influence on changing results in 402 consecutive thoracoabdominal aortic aneurysm repairs. Eur J Cardiothorac Surg 2004;25:701-7. [PubMed]
- Chiesa R, Tshomba Y, Melissano G, et al. Is hybrid procedure the best treatment option for thoraco-abdominal aortic aneurysm? Eur J Vasc Endovasc Surg 2009;38:26-34. [PubMed]
- Chiesa R, Tshomba Y, Logaldo D, et al. Hybrid repair of aortic aneurysms and dissections: the European perspective. Tex Heart Inst J 2011;38:687-90. [PubMed]
- Tshomba Y, Bertoglio L, Marone EM, et al. Visceral aortic patch aneurysm after thoracoabdominal aortic repair: conventional vs hybrid treatment. J Vasc Surg 2008;48:1083-91. [PubMed]
- Greenberg RK, Lytle B. Endovascular repair of thoracoabdominal aneurysms. Circulation 2008;117:2288-96. [PubMed]
- Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2008;47:6-16. [PubMed]
- Cho JS, Carr JA, Jacobsen G, et al. Long-term outcome after mesenteric artery reconstruction: a 37-year experience. J Vasc Surg 2002;35:453-60. [PubMed]
- Rancic Z, Mayer D, Pfammatter T, et al. A new sutureless telescoping anastomotic technique for major aortic branch revascularization with minimal dissection and ischemia. Ann Surg 2010;252:884-9. [PubMed]
- Donas KP, Pecoraro F, Torsello G, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg 2012;55:659-65. [PubMed]
- Pecoraro F, Pfammatter T, Mayer D, et al. Multiple periscope and chimney grafts to treat ruptured thoracoabdominal and pararenal aortic aneurysms. J Endovasc Ther 2011;18:642-9. [PubMed]





