Usefulness of new imaging methods for assessment of type B aortic dissection
Current problem of risk stratification
The current management of uncomplicated type B aortic dissection is medical treatment with antihypertensive medication, with approximately 90% of patients surviving to hospital discharge. However in the longer term, at 5 years, up to 50% of patients in some series have died (1). The latest results from the ADSORB (in press) and INSTEAD-XL have shown a beneficial effect in terms of aortic remodelling with the insertion of a stent graft in these uncomplicated patients (2). Some authorities have suggested that all patients with uncomplicated type B aortic dissection should be treated with an endovascular device, but this is not justified, due to the risk of adverse events such as death, stroke and paraplegia.
The widespread use of 3-dimensional (3D) imaging techniques such as computed tomography (CT) has increased dramatically in the last decade, and with this, the rate of diagnosis of aortic dissection has also increased. We now have a new appreciation of the anatomical complexity of this disease and its unpredictable clinical course. The heterogeneity of this disease has made designing randomised controlled trials with adequate statistical power a challenge, which to date has not been overcome. However, endovascular treatment is not a solution for all patients. There is a sub-group of patients who, despite initially successful endovascular treatment, develop complications such as retrograde type A dissection (sometimes late in the disease course), continued perfusion with expansion of the false lumen and death due to aortic rupture (2).
Current methods to risk stratify patients with type B aortic dissection rely upon static imaging (image data is acquired without reference to the cardiac or respiratory cycle), usually CT angiography. Accepted indications for endovascular intervention in the acute phase include: rupture, malperfusion and refractory pain and hypertension despite maximal medical therapy. In the chronic phase intervention is based upon a maximum aortic diameter ≥5.5 cm, a yearly increase in aortic diameter of ≥1 cm and extension of the dissection, or new symptoms of malperfusion (3,4). Patients with partial thrombosis of the false lumen are thought to be at higher risk of death compared with patients with a patent or completely thrombosed false lumen (5). Other high risk findings on CT imaging include an entry tear on the inner concave curvature of the distal aortic arch compared to one on the outer convex curvature; a total aortic diameter >4 cm; a false lumen diameter ≥22 mm at presentation; the size of the primary entry ≥10 mm; multiple false lumens; and finally visceral branches that are perfused from the false lumen (6-11).
Critical appraisal of these findings shows them to be wanting. For instance: an initial aortic diameter less than 4 cm is now thought to be predictive of a poor outcome whereas previously a diameter greater than 4 cm had been associated with adverse outcomes (12). It is unrealistic to expect a patient with a 22 mm false lumen to behave differently to a patient with a 21 mm false lumen. Furthermore, while it is widely accepted that the error of measuring aortic diameter is in the range of 4 mm, recent publications suggest that an increase in aortic diameter of >4 mm is indicative of rapid growth and is an indication for endovascular stent graft placement (4,13). Further complicating this parameter is the fact that diameter measurements of the aorta can be very difficult to obtain at exactly the same anatomical position on subsequent surveillance CT imaging, and that measurements taken from fixed anatomical points may not represent the site of maximal aortic expansion. Volume measurements are now regarded as being more useful in assessing changes in aortic dimensions, but these are usually not performed in clinical practice (14). New imaging methods are now available which allow us to understand aortic dissection by providing information regarding the underlying hemodynamic and biomechanics of the dissection, which are different in each patient.
Assessment of false lumen thrombus
The amount of thrombus in the false lumen is an important clinical measurement and has been used as a major predictor of prognosis. False lumen thrombosis has been used in both the ADSORB and the INSTEAD randomised trials as a primary and secondary endpoint respectively. The amount of false lumen thrombus is usually assessed by CT using first pass imaging where data acquisition is timed according to the arrival of the contrast bolus in the proximal undissected aorta, most commonly the ascending aorta. Serial images are acquired of the dissected aorta and the absence of contrast agent in the false lumen is assumed to be due to the presence of thrombus. However, flow rates in the false lumen are highly variable and can be very slow and if the primary entry tear is in the distal thoracic aorta. In such a case, the contrast agent may not have retrogradely filled the whole of the patent false lumen before the images are acquired. Interestingly, there is no established CT imaging protocol which is routinely used internationally in assessing patients with type B aortic dissection. Many centres do not routinely perform delayed scans which may more accurately assess the amount of false lumen thrombus.
In a small study, we compared first pass imaging techniques using both CT and MR with delayed phase MR imaging using a blood pool contrast agent (gadofosveset trisodium), which equilibrates throughout the patent false lumen within 10 minutes of injection (15). The blood pool contrast agent binds to serum albumin and remains within the intravascular space for up to an hour. The amount of contrast enhancement in the false lumen is therefore unrelated to the intra-aortic and local flow conditions. The results showed that first pass imaging techniques overestimated the volume of false lumen thrombus by 5-6 times. The results were confirmed by direct thrombus imaging, which confirmed the full distribution of blood pool agent throughout the patent false lumen. Although the study was small, the findings were consistent in every patient.
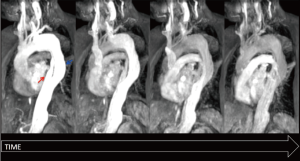
In Tsai’s original paper the finding of partial false lumen thrombosis could be related to ‘mixing’ of the contrast agent within the false lumen and therefore be related to other features such as complex flow patterns (5). It is likely that some patients in the thrombosed group had low flow in the false lumen so that almost no contrast agent had entered the false lumen when the images were acquired (Figure 1). Lack of contrast in the false lumen does not reliably indicate the presence of thrombus when first pass imaging techniques are used. In the future, any randomised trials should use MR imaging with blood pool contrast agents to accurately measure the amount of false lumen thrombus, if this is an important end point of the study. The fact that MR can identify the presence of thrombus by direct imaging suggests that MR has the potential to be used as the sole contrast-agent-free technique to quantify thrombus. However, at present, this technique is prone to artefact and limited by low spatial resolution.
Measuring hemodynamic in the false lumen
The changes in aortic dimensions over time, captured by static three dimensional imaging of aortic diameter or volume, reflect the changes in the underlying hemodynamic and biomechanical conditions in the false lumen. Studies using computer modelling have shown that hemodynamic parameters such as flow pattern, volume and velocity have a role in false lumen expansion and aneurysm formation. These can be directly measured and visualised with MR imaging. Four dimensional phase contrast MR imaging (4D PC-MRI) provides measurements in three spatial dimensions over time. These acquisitions can acquire velocity information over the entire thoracic aortic volume over time so that blood flow in both the true and false lumens can be visualised and quantified. This data is acquired with a respiratory navigator and electrocardiogram, which trigger to reduce respiratory and cardiac motion artifacts respectively. We performed a study to assess the accuracy of these measurements and to observe the hemodynamic features which were related to the rate of aortic expansion (16). The accuracy was assessed by comparison of 4D PC-MRI to the reference standard, 2D PC-MRI, and good correlation and agreement was seen.
Analysis of the hemodynamic features revealed that the stroke volume was greater in the true lumen than in the false lumen and the majority of flow in the true lumen was forwards, whereas there was a high proportion of retrograde flow in the false lumen. The rate of aortic expansion correlated with the measurement of false lumen stroke volume and velocity. One finding of note was that the hemodynamic parameters of the aortic false lumen were different in each patient. This underlines the fact that every patient with aortic dissection is unique, and that understanding the hemodynamic forces is paramount and idiosyncratic to the individual patient.
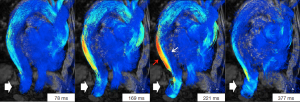
The morphology of the false lumen thrombosis appeared to be related to the pattern of blood flow, but there was no difference in the rate of false lumen expansion between those patients with and without false lumen thrombosis. This study again casts doubt on the significance of false lumen thrombus as a marker for aortic expansion. 4D PC-MRI also identified entry tears, which were on average two per patient. The position of the dominant entry tear in each patient was associated with the area of greatest false lumen expansion, which was higher with distal compared to proximal entry tears. Helical or spiral flow was significantly related to false lumen expansion (Figure 2). 4D MR imaging findings of the altered flow patterns associated with a bicuspid aortic valve have shown that the degree of opening of the valve is directly related to the amount of aortic expansion of the ascending aorta (17). Reduced blood circulation may cause changes in wall shear stress that may progress to affect the vessel wall via hypoxia and endothelial-mediated reactions. The helical flow patterns seen in patients with aortic dissection may be acting through similar mechanisms.
The findings of both of these small studies now need to be reproduced in a much larger series of patients to confirm the findings.
Summary
We have shown that aortic dissection is a complex, idiosyncratic disease which defies attempts to be classified into simple groups. New functional imaging techniques offer the possibility of understanding the intra-aortic biomechanic and hemodynamic environment to offer a means to accurately risk stratify patients with type B aortic dissection. These methods may allow those patients at high risk of aortic expansion to have close surveillance and the opportunity to be treated endovascularly early in the disease course.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Acosta S, Blomstrand D, Gottsäter A. Epidemiology and long-term prognostic factors in acute type B aortic dissection. Ann Vasc Surg 2007;21:415-22. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Kaya A, Heijmen RH, Rousseau H, et al. Emergency treatment of the thoracic aorta: results in 113 consecutive acute patients (the Talent Thoracic Retrospective Registry). Eur J Cardiothorac Surg 2009;35:276-81. [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [PubMed]
- Loewe C, Czerny M, Sodeck GH, et al. A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes complicated, or remains uncomplicated. Ann Thorac Surg 2012;93:1215-22. [PubMed]
- Marui A, Mochizuki T, Mitsui N, et al. Toward the best treatment for uncomplicated patients with type B acute aortic dissection: A consideration for sound surgical indication. Circulation 1999;100:II275-80. [PubMed]
- Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804. [PubMed]
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41. [PubMed]
- Sueyoshi E, Nagayama H, Hayashida T, et al. Comparison of outcome in aortic dissection with single false lumen versus multiple false lumens: CT assessment. Radiology 2013;267:368-75. [PubMed]
- Qin YL, Deng G, Li TX, et al. Risk factors of incomplete thrombosis in the false lumen after endovascular treatment of extensive acute type B aortic dissection. J Vasc Surg 2012;56:1232-8. [PubMed]
- Jonker FH, Trimarchi S, Rampoldi V, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg 2012;94:1223-9. [PubMed]
- Cayne NS, Veith FJ, Lipsitz EC, et al. Variability of maximal aortic aneurysm diameter measurements on CT scan: significance and methods to minimize. J Vasc Surg 2004;39:811-5. [PubMed]
- Stanley GA, Murphy EH, Knowles M, et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg 2011;54:985-92; discussion 992. [PubMed]
- Clough RE, Hussain T, Uribe S, et al. A new method for quantification of false lumen thrombosis in aortic dissection using magnetic resonance imaging and a blood pool contrast agent. J Vasc Surg 2011;54:1251-8. [PubMed]
- Clough RE, Waltham M, Giese D, et al. A new imaging method for assessment of aortic dissection using four-dimensional phase contrast magnetic resonance imaging. J Vasc Surg 2012;55:914-23. [PubMed]
- Hope MD, Sigovan M, Wrenn SJ, et al. MRI hemodynamic markers of progressive bicuspid aortic valve-related aortic disease. J Magn Reson Imaging 2013; [PubMed]





