Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III
Introduction
Atrial fibrillation (AF) is an important cause of stroke. It is well established that most strokes in patients with AF are cardio-embolic, originating from the left atrial appendage (LAA). Three main approaches to stroke prevention in AF are: (I) elimination of AF; (II) prevention of clot formation with antiplatelet or anticoagulant agents; and (III) physical elimination of the LAA which excludes the site of clot formation. Since no AF therapy is able to suppress AF episodes completely, elimination or suppression of AF has not been effective against stroke. Antithrombotic medical therapy has been effective, but is limited by the risk of serious bleeding and by problems with maintenance therapy, including non-issuance of prescriptions, poor compliance, sub-optimal anticoagulation control and physician treatment termination. Accordingly, anticoagulation is suboptimal in many patients and contra-indicated in others. Occlusion or removal of the LAA is logical and recent positive results from a small trial of device closure are promising. However, LAA occlusion or removal has not been thoroughly investigated, and has not been assessed in combination with concomitant procedures. AF is common in patients requiring cardiac surgery and concomitant LAA removal is a small additional procedure with a low intrinsic likelihood of complications.
A large randomized trial to test the hypothesis that opportunistic surgical removal of the LAA at the time of other routine cardiac surgery can reduce long term stroke in patients with AF is important. The implications of a sufficiently powered study, if positive, are to provide conclusive evidence of the efficacy for a logical approach to AF-related stroke and to greatly stimulate the agenda for further research in this promising area. Since the procedure is simple and rapid, procedure specific morbidity is important if large-scale uptake were to occur.
Rationale
Evidence that LAA clot in AF causes embolic stroke
Clinical and diagnostic imaging evidence indicates that at least 70% of all strokes in patients with AF are cardio-embolic from the left atrium (1) and 90% of these arise from the LAA in data from echocardiographic and autopsy studies (2). The LAA has pulsatile flow in sinus rhythm which prevents stasis and thus prevents clot formation. However this function is lacking in AF patients and results in greatly reduced appendage emptying. This stasis, together with increased atrial fibrosis and dilatation typical of AF, and activation of blood coagulation, underlie thrombus formation in AF (Virchow’s triad). Removal or occlusion of the LAA excludes the appendage from the circulation, thus preventing thrombus formation and so prevents embolization (3). Although the atrial appendage is the main source of atrial natriuretic peptide, which plays a role in salt and water homeostasis, a small randomized study (n=77) suggested no adverse effects from LAA removal (4).
There is currently no adequately powered randomized trial of LAA removal or occlusion has been published. The PROTECT AF (n=707) trial was reported last year. The Watchman device, which is designed to occlude the LAA by delivery of an occluding device through a trans-septal approach, was compared to warfarin in an unblinded non-inferiority trial using a composite outcome that included bleeding, thrombotic and fatal outcomes (5). The results showed non-inferiority to warfarin, but due to design criticisms (small size, unconventional primary outcome and wide non-inferiority margins), regulatory approval has been slow to follow. A follow-up study, PREVAIL (n=407), has been reported but not yet published. It demonstrated increased safety of device implantation compared to the PROTECT AF experience, but failed to meet criteria for non-inferiority with respect to efficacy outcomes. These trials have provided some proof-of-concept to the occlusion approach but will continue to be limited by the complexity of the non-inferiority design against effective active therapy (warfarin). Recent non-inferiority trials of new oral anticoagulants (OACs) against warfarin have required enrolments of between 14,000 and 20,000 patients to demonstrate non-inferiority. The rationale for device closure of the LAA as an isolated procedure, with its attendant procedural risks, may be reasonably argued to have comparable risks to warfarin therapy. However, if the LAA occlusion can be performed at the same time of routine cardiac surgery, then this procedure can be performed with almost no additional risk. Thus it is reasonable in AF patients undergoing routine cardiac surgery to occlude the LAA and continue with postoperative oral anticoagulation. LAA occlusion and antithrombotic therapy have completely different mechanisms of stroke prevention; occlusion removes the anatomic location for most potential cardiac thrombi, while antithrombotic therapy reduces the tendency for thrombi formation. Even the most effective antithrombotic therapy needs to be taken once or twice every day over years (even decades) to be fully beneficial; a challenge even to the most compliant patient. LAA occlusion once adequately performed will never re-form and thus will provide un-interrupted protection against thrombus formation, and potentially stroke, for life. It is a strong hypothesis that the two approaches will be additive or synergistic against stroke.
Evidence that oral anticoagulation reduces embolic stroke
OAC therapy reduces the risk of stroke in AF and is recommended for stroke prevention in patients with AF who have risk factors (6). A Cochrane meta-analysis that included 29 trials and 28,044 patients (7,8) reported that warfarin reduced the relative risk of stroke by 64% (95% CI, 49% to 74%) compared to no treatment and by 37% (95% CI, 23% to 48%) compared to aspirin. Aspirin is also effective, reducing the relative risk of stroke in AF by 20%. Anticoagulation is now recommended for all higher risk patients with AF. However, there are still many patients who receive antiplatelet therapy alone. Administrative database surveys indicate that only about two-thirds of patients who might benefit from anticoagulant therapy actually receive OAC, and discontinuation rates of warfarin approach 50% by three years (9).
New OACs are being introduced which also reduce stroke in AF, including the direct thrombin inhibitor, dabigatran, and the Factor Xa inhibitors rivaroxaban and apixaban. These agents have been evaluated in large clinical trials and have been shown to be non-inferior, and in some cases superior, to warfarin for stroke reduction; with similar or less bleeding. Dabigatran 110 mg, apixaban and rivaroxaban all showed very similar rates of ischemic stroke relative to warfarin, whereas dabigatran 150 mg showed a significant 25% relative risk reduction (RRR) compared to warfarin. Both Factor Xa inhibitors and both dabigatran doses showed a large reduction in hemorrhagic strokes compared to warfarin. Major bleeding rates on all these agents, however, exceeded 3% per year, and minor bleeding rates were over 10% per year. Thus hemorrhage remains a significant limitation of both old and new OACs. One advantage of the new agents is that they do not require monitoring, which makes them easier to take than warfarin; but this paradoxically limits the physician’s ability to ensure patient compliance.
Limitations of OAC which LAA occlusion may mitigate
There are many limitations to OAC therapy: (I) increased risk of bleeding; (II) need for monitoring of coagulation (International Normalized Ratio, INR) for warfarin; (III) patient non-compliance; (IV) physician reluctance to prescribe, especially to elderly patients; and (V) frequent need for therapy discontinuations for surgery, procedures and diagnostic tests.
Increased bleeding, both major and minor, is inherent in all antithrombotic therapy. For example, in the recent RE-LY trial, the annual rates of major bleeding were 3.4%, 2.7% and 3.1% for dabigatran 110 mg BID, 150 mg BID and warfarin, respectively; and minor bleeding rates were 13%, 15% and 16% per year. In both ACTIVE and RE-LY trials, major bleeding increased the adjusted risk of death compared to those without bleeding [hazard ratio (HR), 4.60 (4.16-5.09)] (10). Even minor bleeding may lead to discontinuation of antithrombotic therapy and exposure to stroke risk. LAA occlusion could provide some protection during OAC discontinuance.
Patient dissatisfaction with the need for monitoring of warfarin therapy is a major limitation of this therapy. Approximately two thirds of patients are reliably maintained within the target INR range at all times, even in clinical trials (11). In typical community practice, the time in therapeutic range falls to about 50% as demonstrated by a recent overview of studies (12,13). A low proportion of time spent in the target INR range is strongly associated with an increased risk of both stroke and bleeding (14). Thus, a concurrent therapy such as LAA occlusion that independently reduces stroke and is continuously effective, is likely to be beneficial in patients receiving warfarin. LAA occlusion would theoretically provide protection to patients when their INR is non-therapeutic.
The lack of INR control may relate significantly to patient non-compliance, which is a major limitation inherent to OAC therapy. In a major review of medication compliance for cardiovascular disease, Ho and colleagues estimated that 25-55% of patients do not take their chronic cardiac medications as prescribed (15). Medication adherence for asymptomatic or chronic conditions is typically lower than that for acute or symptomatic conditions, and drops substantially after the initial months of therapy (15-18). The reasons for this include patient-related factors (e.g., health illiteracy, forgetfulness, socio-economic barriers), medication-related factors (e.g., cost, complexity of the regimen, side effects) and provider-related factors (e.g., a lack of coordinated care and follow-up) (18-21). Non-adherence is strongly skewed towards under- rather than over-dosing, and is associated with an increased risk of death, disability, hospitalization, and avoidable health care costs (15,22-25). A recent study of point-of-care testing in 53 Australian general practices is instructive. The study included patients who required OAC, and only 43% of patients on anticoagulants reported consistent adherence to therapy during the study (26). There is also substantial evidence that physicians under-estimate the degree of medication non-compliance even in patients who they ‘know well’ (27). Compliance issues continue to be a problem with all medications and may be more of a problem with new anticoagulants than with warfarin, due to short half-lives and lack of need for regular monitoring. LAA occlusion could benefit for many patients on medical therapy who are sometimes non-compliant.
The avoidance or under-use of anticoagulants is widely documented in virtually every country where this has been studied (28-34). Many patients (up to 50%) are unsuitable for warfarin for a variety of reasons and some will remain unsuitable for the new anticoagulants. In the CCORT AF study, using prescription claims databases in Alberta, British Columbia and Ontario from 1997 to 2000, less than one-half of AF patients filled a prescription for warfarin within 90 days of discharge for an AF hospitalization (32). After initiation of warfarin, discontinuation is very common. In one large administrative database registry from the United Kingdom, Gallagher et al. reported warfarin discontinuation rates of 50% within a 4-year follow-up period (9). A very recent analysis of Ontario Drug Benefit claims data in 125,195 patients >65 years with AF who initiated warfarin therapy, found that almost one third (31.8%) discontinued warfarin within one year of initiation, and the median time to discontinuation was 2.9 years (35). The main limitation of warfarin is concern about bleeding, and this often prevents its use in otherwise suitable patients (36,37). This suggests that even with the new anticoagulants, non-use and discontinuation of anticoagulants will be a problem; one that can be mitigated potentially by LAA occlusion.
Interruption of anticoagulant therapy for surgery, procedures and diagnostic tests is very common in patients with AF (38). This creates a window of risk to patients, in addition to the risk of failure of re-initiation of therapy after discontinuation. LAA occlusion can potentially be very useful in this situation.
AF is associated with a systemic hypercoagulable state, where platelet function is enhanced with increased plasma levels of thromboglobulin and platelet factor 4. Systemic markers of activation of the coagulation cascade, such as thrombin-antithrombin II complex, D-dimers, fibrinogen, and prothrombin fragments 1 and 2, are also increased. Although most thrombi form in the LAA, some likely come from aortic plaque, the left ventricle and elsewhere. Thus a systemic antithrombotic therapy is likely a very good complement to a focused surgical intervention that targets only one source of embolism, albeit the most important one.
Miscellaneous evidence regarding LAA occlusion
Prior to the publication of PROTECT AF, the literature was dominated by observational studies and formed the basis of the American Heart Association recommendation to occlude the LAA in AF patients undergoing mitral valve surgery (39,40). In a retrospective study examining 205 patients post mitral valve surgery, the success rate of LAA closure approached 90%. Multivariate analysis demonstrated the absence of LAA ligation as an independent predictor of occurrence of an embolic event [odds ratio (OR) 6.7; 95% CI, 1.5-31.0]. Results from case series of ablation procedure patients are also often cited to support the amputation of the LAA (41). The Maze procedure attempts to eliminate AF through a series of cuts in the right and left atria, suturing them closed, and excising both atrial appendages in a similar fashion. Cox et al. published a case series of 306 patients who underwent a “cut and sew” Maze procedure (42). Rates of stroke were low but the majority of patients (n=162) were very low risk. Furthermore, from this study it is impossible to determine whether the stroke protection, if real, was from rhythm control or LAA exclusion.
Conversely, some investigators have reported unfavorable outcomes after surgical LAA exclusion. One such study by Almahameed et al., involving 136 patients undergoing LAA amputation at the time of mitral valve surgery (43) demonstrated that during a mean follow up period of 3.6 years, 15% of the patients who underwent LAA amputation, but did not receive warfarin, had thromboembolic events. In the group that underwent LAA amputation and received warfarin at discharge, only 10% had thromboembolic events. A regression analysis on this cohort revealed that warfarin, not LAA amputation, resulted in reduced stroke risk. Another paper by Bando et al. (n=812) investigating the risk factors for stroke in patients undergoing mitral valve surgery, demonstrated in subgroup analysis that closure of the LAA (n=47) did not have a significant effect on the incidence of stroke in patients with AF (P=0.69) (44). Additionally, the group performed a univariate analysis on 812 patients (78% with AF), which showed that LAA closure was not significant for preventing late (eight years) stroke.
It is emphasized that these and several other small observational studies do not have sufficient power or freedom from sample bias to provide the level of evidence needed to clearly answer this important question.
Trial design
Left Atrial Appendage Occlusion Study (LAAOS) III is a prospective, double-blind, international multicenter, randomized blinded trial with adjudicated outcome assessments, comparing concomitant surgical LAA occlusion to no occlusion in patients with AF/flutter who are undergoing routine cardiac surgery. The target recruitment is 4,700 patients. The primary hypothesis is that LAA occlusion will reduce stroke or systemic embolic events compared to no occlusion over the period of follow-up (mean four years), with OAC therapy recommended in both arms.
Eligible and consenting patients will be randomized via the central interactive web randomization system (IWRS) at the Population Health Research Institute, Canada. Each patient will be assigned in a blinded fashion to one of two groups (LAA occlusion or no LAA occlusion) according to a computer generated randomization list. Patients will be considered randomized when the intervention allocation has been provided through the IWRS. The confidential allocation email will be sent to the participating surgeon (and not to the research team) to maintain blinding of all others associated with the study.
All patients will be followed from the time of randomization until the final follow-up visit. Following randomization and baseline data collection, visits will occur at hospital discharge, 30 days, one year and annually thereafter until the common study end date (approximately five years after the first patient randomized). Interim telephone calls will be held at the 6-month intervals to maintain contact with the patients.
Patient selection
Patients undergoing cardiac surgery requiring the use of cardiopulmonary bypass are included if they: (I) ≥18 years; (II) have a documented history of AF or flutter; (III) have a CHA2DS2-VASc score ≥2; and (IV) provide written informed consent.
p>Patients are excluded if they are undergoing (I) off-pump cardiac surgery; (II) heart transplant; (II) complex congenital heart surgery; (IV) isolated ventricular assist device insertion; (V) redo cardiac surgery; (VI) mechanical valve implantation. Furthmore, patients who have had a previous placement of a percutaneous LAA closure device are excluded.Study intervention
The intervention under investigation is surgical occlusion of the LAA compared to no LAA occlusion. The trial will permit the following techniques of LAA occlusion: (I) amputation of the LAA and closure; and (II) stapler closure of the LAA. The preferred technique is amputation and closure as demonstrated by the video found at http://www.youtube.com/watch?v=aPoeoAhIjGw. Surgical devices to close the appendage will be considered for use in the trial by the steering committee, based upon the available evidence for the successful occlusion of the LAA.
Intraoperative transesophageal echocardiography (TEE) is encouraged to determine successful closure of the appendage. Successful occlusion is defined as TEE Doppler assessment demonstrating an absence of flow across the suture line and a stump of
Study outcomes
The primary outcome is the first occurrence of stroke or systemic arterial embolism over the duration of follow-up.
The secondary outcomes over the duration of follow-up (unless otherwise specified) are: (I) total mortality; (II) operative safety outcomes (chest tube output in the first post-operative 24 hours, rate of post-operative re-exploration for bleeding in the first 48 hours post-surgery and 30-day mortality); (III) re-hospitalization for heart failure; (IV) major bleeding; and (V) myocardial infarction.
Definitions of study outcomes can be found on the online supplement.
Sample size and statistical considerations
This study will enroll 4,700 patients with a mean follow-up of four years, which will allow us to detect a 25% RRR in the primary outcome with an expected control event rate of 2.5% per year. This trial would have 80% power, accounting for a 2%/year loss of patients due to competing death. This sample size is contingent on reasonable assumptions about the patient risk and the types of antithrombotic therapy that patients will receive during follow up. If the event rates are lower than expected, follow-up can be extended with this study design.
The enrollment requirement of this trial depends primarily on two parameters: the expected event rate in the control arm and the treatment effect expected from LAA occlusion. We have estimated the event rate in the control arm from the event rates on various antithrombotic treatments in recent trials (Table 1) with similar CHADS2 score to what is expected. We have performed a registry of 1,886 patients in which we observed that the mean CHADS2 score of patients with AF coming to cardiac surgery was 2.3.
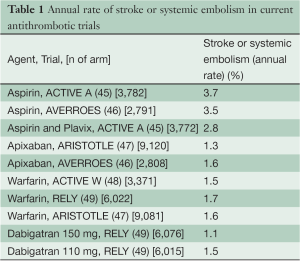
Full table
Table 2 shows the expected treatment effects of LAA occlusion in different sub-groups of patients expected to be enrolled into the study. As can be appreciated from Table 2, to properly estimate the control event rate we also need to estimate the rate of use of different antithrombotic medications during follow-up. Numerous surveys indicate that OACs are used in approximately 50% to 60% of high-risk patients with AF due to difficulties with controlling INR, bleeding risk, patient reluctance and physician behavior. The use of OACs will tend to increase over the next few years as the new anticoagulants are introduced; however, there will still remain a substantial number of patients who either take aspirin or no therapy due to refusal to take an anticoagulant, difficulty with INR management, high cost of new anticoagulants, and/or development of renal failure which increases the risk of anticoagulation. Therefore we estimate that the number of patient-years of follow on antiplatelet or no antithrombotic therapy will be 35%±5%. We have very good estimates of the rate of stroke or systemic embolism for these patients from ACTIVE A and AVERROES (3.7% per year on aspirin and 5.1% on no antithrombotic therapy). Because of cost issues and familiarity, we estimate that warfarin and other Vitamin K antagonists will remain the most common OACs used (45% of patient-years of follow up). There will be gradually increasing use of dabigatran and the Factor 10a inhibitors over the next five years. It is estimated that 20% of patient years of follow-up will be on dabigatran, rivaroxaban, or apixaban. We estimate, based on the recent large trials, that the primary event rate in control patients taking warfarin will be 1.7% per year, and in those taking one of the new anticoagulants it will be 1.5% per year. Thus the overall annual event rate in the control arm without LAA occlusion is estimated at 2.5% per year.
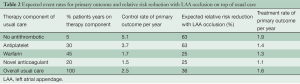
Full table
This study is 80% powered to detect a 25% RRR. A 25% treatment effect is reasonable because the PROTECT AF trial of device closure suggests that the effect of LAA occlusion is similar to that of warfarin, although the mechanism is obviously different and the effect of LAA occlusion will be additive to that of medical therapy. Table 2 shows that the largest effect will likely occur in those receiving no therapy or aspirin. The most recent data comparing an OAC to aspirin in AF patients comes from AVERROES, where the reduction in ischemic stroke with apixaban compared to aspirin was 63% [HR, 0.37; 95% CI, 0.25-0.55] P
Outcome analysis
The intention-to-treat principle, in which all participants will be included in their assigned treatment groups regardless of actual surgical procedure performed, will guide all analyses. A time-to-event analysis will be used to test the primary outcome variable. The primary outcome (stroke or systemic arterial embolism) will be presented using Kaplan-Meier survival curves, and the treatment effect as measured by the HR and 95% confidence interval will be derived by the Cox proportional hazards model. A P-value of t-test, chi-square test, or non-parametric tests where appropriate. The primary outcome will be analyzed at a mean follow-up of four years.
Planned subgroup analyses
Additional Cox models will be used to evaluate interactions between treatment and subgroups of interest: antithrombotic used, CHA2DS2-VASc score, left atrial (LA) dimension, rheumatic heart disease, and atrial ablation procedure. The primary analysis will be repeated secondarily as a per protocol analysis.
Data safety monitoring board (DSMB)
The independent DSMB will undertake two formal interim analyses when 50% (188 events) and 75% (282 events) of the expected events have occurred. Conservative statistical guidelines for data monitoring have been developed and will follow the modified Haybittle-Peto rule. For efficacy, reductions in events of ≥4 SD in the first interim analysis and ≥3 SD in the second will be used. The DSMB in making a recommendation for early stopping will also consider the consistency of the secondary endpoints and any relevant external data.
Vanguard phase
The study has begun with a vanguard phase that is funded by the Canadian Institutes for Health Research (CIHR). The vanguard phase has an identical design to the full trial and is planned to validate both general feasibility and key design assumptions. Specifically, blinded analysis after 300 patients have been recruited will be performed to validate (I) feasibility of recruiting two patients per month in each of 12 centers globally; (II) the assumption that in the year following surgery, 30% of randomized patients will be managed on antiplatelet therapy alone and 65% will be on oral anticoagulation; and (III) the assumption that the rate of successful surgical LAA occlusion will be ≥90%. Enrolment of 300 patients will provide the following estimates within the listed margin of error, 95% of the time: (I) antiplatelet therapy alone will be used in 30%±5.2% and OAC use in 65%±5.4%; (II) the rate of successful conclusion is at least 90%±3.4%; (III) the rate of successful recruitment will be achieved in 90%±16% of sites.
Discussion
Despite the first description of surgical LAA occlusion for prevention of arterial emboli in AF being published over half a century ago (50), the field of cardiac surgery has yet to provide definitive evidence that this approach is an effective strategy for stroke prevention. There is a great need to explore non-medical approaches to stroke prevention in AF that are likely to be complementary to antithrombotic therapy, or which could, in some patients, replace medical therapy. The present trial addresses the potential of LAA occlusion at the time of cardiac surgery to reduce stroke from AF as an adjunctive therapy, over and above the antithrombotic therapy that the patient will continue to receive. This study will be the only study which is sufficiently powered and prospectively randomized to consider this hypothesis. AF is a major health concern and continues to grow in its burden. AF is present in about one sixth of patients admitted with stroke and is seen in 10% of patients coming to cardiac surgery, during which time it is safe to remove this documented site of thrombus formation. There are in excess of two million cardiac surgical procedures performed annually world-wide, and the impact of this minimal-cost, low-risk procedure could be immense.
We have chosen the proposed design for the following reasons: (I) the proposed trial answers the most relevant clinical question for the surgeon operating on a patients who happens to have AF; (II) a blinded superiority trial will provide a more compelling answer to the question of whether LAA occlusion prevents stroke than an unblinded non-inferiority trial; (III) compared to a trial of LAA occlusion versus warfarin, the proposed trial will be much less challenging to execute; (IV) patients are more likely to enroll as they do not have to forgo a proven effective therapy if randomized to LAA occlusion; (V) surgeons will find the trial less burdensome as the intervention is completed quickly, follow-up is easy and there are no compliance issues; (VI) compliance to no anticoagulant therapy in the LAA occlusion arm would be very difficult given the massive density of information about the merits of OACs in the literature, in the National Guidelines, from the pharmaceutical industry and in the lay press; and (VII) the trial of LAA occlusion versus could only be blinded with great difficulty and cost. A properly designed non-inferiority trial of LAA occlusion versus warfarin would be very large and expensive (15,000 patients). LAAOS III will be a definitive trial, answering whether occlusion of the LAA provides protection against stroke.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Supplement: study outcome definitions
Stroke
Diagnosis of stroke will require new focal neurological symptoms with rapid onset, lasting at least 24 hours. All strokes will be classified as definite ischemic, definite hemorrhagic or type uncertain.
Systemic arterial embolism
Systemic arterial embolism will be judged to occur where there is a clinical history consistent with an acute loss of blood flow to a peripheral artery (or arteries), which is supported by objective evidence of embolism.
Major bleed
Major bleeding within the first 48 hours of surgery is defined as per BARC Type 4: (I) perioperative intracranial bleeding within 48 hours; and/or (II) reoperation after closure of sternotomy for the purpose of controlling bleeding; and/or (III) transfusion of ≥5 units whole blood or packed red blood cells within a 48 hour period (note: cell saver products are not counted); and/or (IV) chest tube output ≥2 L within a 24-hour period.
Major bleeding after 48 hours of surgery is defined as per ISTH: (I) fatal bleeding; and/or (II) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome; and/or (III) bleeding causing a fall in hemoglobin level of 2.0 g/dL or more, or leading to transfusion of two or more units of whole blood or red cells.
Hospitalization with heart failure
Re-hospitalization with an overnight stay or prolongation of an existing hospitalization due to heart failure which requires both clinical (i.e., any of the following signs: elevated jugular venous pressure, respiratory rates, crepitations, or presence of S3) and radiographic evidence (e.g., vascular redistribution, interstitial pulmonary edema, or frank alveolar pulmonary edema).
Myocardial infarction (MI)
Perioperative MI (48 hours) is defined as ischemic symptoms; ECG changes consistent with myocardial infarction (new significant Q waves in two contiguous leads) or evolving ST-segment or T-wave changes in two contiguous leads signifying ischemia or new LBBB; or ST segment elevation and elevated cardiac markers (troponins or CK-MB) in the necrosis range. Myocardial injury occurring after a percutaneous coronary intervention (PCI) is included in the late perioperative MI group but is defined as elevation of cardiac markers at least three times upper limit of normal (ULN) within 24 hours of PCI , or the characteristic evolution of new ECG changes.
References
- Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 1991;84:527-39. [PubMed]
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9. [PubMed]
- Leung DY, Black IW, Cranney GB, et al. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol 1994;24:755-62. [PubMed]
- Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93. [PubMed]
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42. [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur Heart J 2006;27:1979-2030. [PubMed]
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [PubMed]
- Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501. [PubMed]
- Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost 2008;6:1500-6. [PubMed]
- Eikelboom JW, Connolly SJ, Hart RG, et al. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol 2013;62:900-8. [PubMed]
- Gottlieb LK, Salem-Schatz S. Anticoagulation in atrial fibrillation. Does efficacy in clinical trials translate into effectiveness in practice? Arch Intern Med 1994;154:1945-53. [PubMed]
- Samsa GP, Matchar DB, Phillips DL, et al. Which approach to anticoagulation management is best? Illustration of an interactive mathematical model to support informed decision making. J Thromb Thrombolysis 2002;14:103-11. [PubMed]
- Lafata JE, Martin SA, Kaatz S, et al. The cost-effectiveness of different management strategies for patients on chronic warfarin therapy. J Gen Intern Med 2000;15:31-7. [PubMed]
- Reynolds MW, Fahrbach K, Hauch O, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest 2004;126:1938-45. [PubMed]
- Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028-35. [PubMed]
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462-7. [PubMed]
- Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA 2002;288:2880-3. [PubMed]
- Baroletti S, Dell’Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455-8. [PubMed]
- Cutler DM, Everett W. Thinking outside the pillbox--medication adherence as a priority for health care reform. N Engl J Med 2010;362:1553-5. [PubMed]
- Oates DJ, Paasche-Orlow MK. Health literacy: communication strategies to improve patient comprehension of cardiovascular health. Circulation 2009;119:1049-51. [PubMed]
- Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107-16. [PubMed]
- Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521-30. [PubMed]
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177-86. [PubMed]
- Parker CS, Chen Z, Price M, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN-RANGE study. J Gen Intern Med 2007;22:1254-9. [PubMed]
- Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772-9. [PubMed]
- Gialamas A, Yelland LN, Ryan P, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Med J Aust 2009;191:487-91. [PubMed]
- Stephenson BJ, Rowe BH, Haynes RB, et al. The rational clinical examination. Is this patient taking the treatment as prescribed? JAMA 1993;269:2779-81. [PubMed]
- Thromboembolic prophylaxis in 3575 hospitalized patients with atrial fibrillation. The Clinical Quality Improvement Network (CQIN) Investigato. Can J Cardiol 1998;14:695-702. [PubMed]
- Grønlund A, Grønlund L, Clevin L, et al. Management of missed abortion: comparison of medical treatment with either mifepristone + misoprostol or misoprostol alone with surgical evacuation. A multi-center trial in Copenhagen county, Denmark. Acta Obstet Gynecol Scand 2002;81:1060-5. [PubMed]
- Caro JJ, Flegel KM, Orejuela ME, et al. Anticoagulant prophylaxis against stroke in atrial fibrillation: effectiveness in actual practice. CMAJ 1999;161:493-7. [PubMed]
- Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med 2004;164:55-60. [PubMed]
- Humphries KH, Jackevicius C, Gong Y, et al. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol 2004;20:869-76. [PubMed]
- Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new-onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol 2006;97:538-43. [PubMed]
- Smith NL, Psaty BM, Furberg CD, et al. Temporal trends in the use of anticoagulants among older adults with atrial fibrillation. Arch Intern Med 1999;159:1574-8. [PubMed]
- Gomes T, Mamdani MM, Holbrook AM, et al. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med 2012;172:1687-9. [PubMed]
- Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000;160:41-6. [PubMed]
- Choudhry NK, Soumerai SB, Normand SL, et al. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. Am J Med 2006;119:607-15. [PubMed]
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012;120:2954-62. [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257-354. [PubMed]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84-231. [PubMed]
- Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am Heart J 2006;151:956-61. [PubMed]
- Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:833-40. [PubMed]
- Almahameed ST, Khan M, Zuzek RW, et al. Left atrial appendage exclusion and the risk of thromboembolic events following mitral valve surgery. J Cardiovasc Electrophysiol 2007;18:364-6. [PubMed]
- Bando K, Kobayashi J, Hirata M, et al. Early and late stroke after mitral valve replacement with a mechanical prosthesis: risk factor analysis of a 24-year experience. J Thorac Cardiovasc Surg 2003;126:358-64. [PubMed]
- ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009;360:2066-78. [PubMed]
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17. [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [PubMed]
- ACTIVE Writing Group of the ACTIVE Investigators, Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [PubMed]
- Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc 1949;140:769-72. [PubMed]





