Safeguards and pitfalls in minimally invasive mitral valve surgery
In experienced hands, minimally invasive mitral valve surgery offers multiple advantages, including far more than improved cosmetic results. The entire range of mitral valve surgeries can be performed using this approach. To achieve the desired result, various key steps throughout the case need to be performed with close attention to detail. Keeping the procedure simple, efficient and with perfect exposure will allow the surgeon to focus upon the task at hand.
Preoperative echocardiography is a crucial investigative modality used to identify the specific underlying mitral valve pathology. Echocardiography is essential in establishing the operative indication, determining patient selection and allowing for pre-operative planning. Valve parameters (size of the orifice and height of the anterior leaflet), annular calcification, size of the left atrium, and thrombus in the left atrial appendage should be evaluated. To prevent myocardial injury, significant aortic valve regurgitation needs to be excluded prior to surgery. Safe application of cardioplegia is not possible in patients with moderate (or severe) aortic regurgitation, and in this instance, a minimally invasive approach should not be pursued.
A pre-operative chest X-ray should be performed to determine the level of the diaphragm. This is important for planning the site of the thoracic incision, with the chest film used to determine the correct height and appropriate intercostal space to enter the thorax.
Previous thoracic surgery or other potential causes of significant pleural adhesions in the right hemithorax should prompt the surgeon to reconsider a minimally invasive approach. In this situation, the increased risks of significant intra-operative hemorrhage (US spelling) and/or bronchopleural fistulas may outweigh the benefits of minimally invasive surgery, and the patient and surgical team should discuss (and be prepared for) the possibility of a conversion into an open sternotomy.
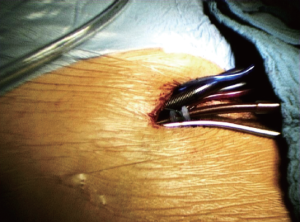
In our practice, we prefer commencing the surgical procedure by placing both the venous and arterial cannulae into the right femoral vessels using the Seldinger technique (Figure 1). Placing cannulation lines out of the surgical field is a major advantage. However, the surgeon must also be aware of potential hazards when preparing the groin. Irritation of the femoral nerve, seromas and lymphatic fistulas should be avoided by using atraumatic techniques with diligent ligation or clipping of sizeable vessels. The incision should be just long enough to allow safe access to both vessels. Prior to cannulation, isolating the femoral artery via blunt dissection will allow safe application of clamps if required. In some situations the artery may require repair after the cannula is removed. The guide wire as well as the two-staged venous cannula should be inserted with its tip in the superior vena cava under transesophageal echo guidance to avoid inadvertently perforating the right atrial wall. Upon placing the arterial cannula, retrograde dissection can be performed. The femoral artery needs to be inspected carefully for plaques prior to placing the guide wire. In patients with significant peripheral artery disease, direct cannulation of the ascending aorta or even the axillary artery should be considered.
Positioning the patient with the right chest wall slightly elevated off the operating table allows for convenient placement of the thoracotomy incision. The thoracotomy incision (approximately 5 cm long) is made straight laterally from a point 1-2 cm inferior and lateral to the nipple in men, and in the sub-mammary crease in women. Identifying the most appropriate intercostal space (usually 4th) depends on the height of the diaphragm, as determined on the preoperative chest X-ray. A more superior and lateral incision allows for a straight on view of the mitral valve. Prior to entering the pleural space, the lungs are taken off the ventilator. Intercostal muscle and vascular hemorrhage can be avoided by staying along the superior surface of the rib. A soft tissue retractor is used to prevent the incidence of fat emboli (when the left atrium is later accessed). The retractor should be opened gradually to avoid rib fractures and the potential for postoperative chest wall hemorrhage or hemothorax.
Fat pads covering the pericardium should be removed sparingly and well cauterized. To avoid damage to the phrenic nerve, the pericardium should be entered 3 cm anteriorly, allowing enough space for placing stay sutures at the posterior edge for lateral traction.
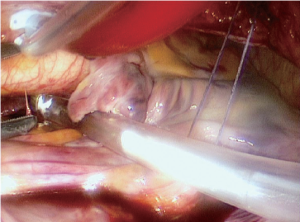
Preparations prior to clamping the aorta include blunt dissection of the oblique sinus (Figure 2) and dissection of the fat in the interatrial groove. When placing the aortic cross-clamp using the Chitwood clamp (1), there is a chance of injury to the pulmonary artery or left atrial appendage, which would force the surgeon to convert to a full sternotomy. Cardioplegia can be utilized using either a rigid large bore cannula or a y-catheter. This allows for repeat cardioplegia doses and standard de-airing maneuvers later on. Other surgical groups have reported using endoaortic balloons for aortic occlusion and cardioplegia application instead of external clamping (2). In our experience, after two previous incidents of aortic dissection caused by endoaortic balloons, we have chosen to abandon this technique. Upon completion of cardioplegia application, while decompressing the left heart through a stab incision in the left atrium, the left atriotomy is extended, with careful attention to avoid injury of the coronary sinus and perforation of the interatrial septum or pulmonary veins. While positioning the retractor to elevate the left atrial roof, there is a risk that the venous cannula may be dislodged out of the SVC, which would compromise the upper body venous drainage. Hence, correct cannula placement and normal central venous pressure need to be confirmed prior to proceeding further.
In re-do scenarios, cross-clamping of the aorta might not be possible due to adhesions at the ascending aorta. Minimally invasive mitral valve surgery using fibrillatory arrest can still be performed in these cases, but only in the absence of concomitant aortic regurgitation (3).
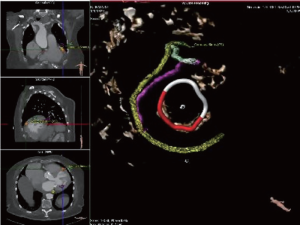
During annuloplasty ring or valve implantation, placement of the sutures should be performed in consideration of the anatomical proximity of the aortic valve and the circumflex artery. Deep stitches in the region of the anterolateral commissure and the A1 segment should be avoided. The distance of the circumflex artery from the mitral annulus is variable, depending on left or right dominance of the coronary system (Figures 3,4).
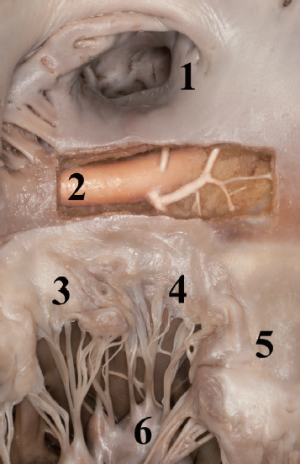
After completion of the mitral valve procedure, the left atrium should be closed in two layers and usual de-airing maneuvers are performed prior to removing the aortic cross-clamp. The potential sites of peri-operative hemorrhage include the trocar site, the anterior chest wall stab incision for the atrial roof retractor close to the mammary vessels, and the soft tissues at the thoracotomy site. These areas should be thoroughly inspected prior to closing the chest to minimize postoperative hemorrhage and take backs. Deflating the lung while inspecting these chest wall sites can be very helpful; hence, our team prefers de-cannulating afterwards. Some groups advocate using double lumen tube intubations routinely with the advantage of de-cannulation and protamine application prior to final bleeding control under isolated left lung ventilation.
Postoperative ECG changes, wall motion abnormalities on echocardiography and impaired hemodynamics may suggest air embolism or compromised circumflex artery perfusion (4). Pre- and perioperative transesophageal echocardiography has proven to be useful in identifying the course and flow of the circumflex artery. If there is suspicion of new onset coronary malperfusion, caused by extrinsic compression of the implanted prosthesis, immediate transfer to the catheterization lab is of utmost importance in initiating the appropriate diagnostic and therapeutic measures promptly. If interventional treatment fails, surgical bypass grafting of the circumflex artery is required as soon as possible to minimize further myocardial damage.
Meticulous attention to detail is required in minimally invasive mitral valve surgery, allowing the surgeon to move through the case with efficiency and optimal results. Particularly devastating complications associated with minimally invasive mitral valve cases include (5): phrenic nerve injury, vascular complications of the femoral artery, aortic dissections, and perioperative strokes. These complications should be minimized at all costs.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chitwood WR Jr, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg 1997;63:1477-9. [PubMed]
- Loforte A, Luzi G, Montalto A, et al. Video-assisted minimally invasive mitral valve surgery: external aortic clamp versus endoclamp techniques. Innovations (Phila) 2010;5:413-8. [PubMed]
- Umakanthan R, Leacche M, Petracek MR, et al. Safety of minimally invasive mitral valve surgery without aortic cross-clamp. Ann Thorac Surg 2008;85:1544-9; discussion 1549-50. [PubMed]
- Calafiore AM, Iacò AL, Varone E, et al. Distortion of the proximal circumflex artery during mitral valve repair. J Card Surg 2010;25:163-5. [PubMed]
- Falk V, Cheng DC, Martin J, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations (Phila) 2011;6:66-76. [PubMed]