The art of arterial revascularization—total arterial revascularization in patients with triple vessel coronary artery disease
Introduction
Since the first series of left internal thoracic artery (LITA) grafts published by Konstantinov (1), the LITA has become the standard treatment for coronary artery bypass grafting (CABG). Barner reported the use of bilateral ITA (BITA) for coronary bypass almost 30 years ago, but was not widely accepted initially (2,3). Tector and others are credited with introducing composite arterial grafting using free ITAs, sequential grafts, T-grafts and combinations for the treatment of multi-vessel coronary artery disease (CAD) (4-7). Many observational studies have suggested that arterial grafting is superior to saphenous vein (SV) techniques, but at present, BITA or multiple arterial grafting have not proved popular for many reasons, mainly because of perceived technical complexity or fear of serious complications such as sternal infection. Few units have reported a BITA grafting rate greater than 10% (8). Survival after bilateral versus single internal thoracic artery (ITA) grafting is being assessed by the randomized controlled Arterial Revascularisation Trial (ART) of Taggart et al. (9).
Nonetheless, in patients with multi-vessel CAD, ongoing SV graft failures have led some surgeons to adopt a policy of extensive or total arterial revascularization using one or both internal mammary arteries and alternative conduits such as the radial artery (RA) (10) or right gastroepiploic arterial grafts (11). Arterial grafts have the advantage of durability and may have a protective effect by reducing the progression of native CAD in grafted vessels (12). Multiple arterial grafting may thereby improve survival in patients receiving total arterial revascularization. Beginning in 1995, total arterial revascularization has been the operation of choice in our unit for treatment of three-vessel CAD, with various iterations or graft configurations in use.
Operative techniques
Conduit planning in patients with triple-vessel disease (TVD)
The success of a surgical procedure is related to careful assessment and planning. All patients should be considered for multiple arterial grafts. Although the BITA rate is about 35% in our unit there are a number of recognized relative contraindications, including obesity (BMI >35), severe airways disease, diabetes, radiotherapy, or immunosuppression. Recent data suggest that the risks of the latter are markedly reduced by the use of skeletonization (13). The RA is our conduit of choice after the ITAs. Most patients with TVD require three major conduits; combined with ITA conduits, our choice is to use the RA rather than a SVG for the supplementary bypass grafts. There are contraindications to RA harvesting: 5% of patients have an abnormal ulnar collateral flow as judged by the Allen test (a return of blood to the ischemic hand in greater than 10 seconds after release of the ulnar pulse), while palpable or visible calcification during harvesting pose potential problems in the elderly. RA trauma following recent cardiac catheterization is a more recent concern, and limited data and anecdotal experience suggests these conduits should be avoided. Patients receiving or likely to receive dialysis may require the preservation of RAs for future fistulae as lack of vascular access remains a major cause of death in long-term dialysis patients.
Aside from the availability of conduits, other factors which may influence optimal planning are the severity of the target lesions and the decision to perform the procedure on- or off-pump. In lesions with less than 70% stenosis in the left circulation, and probably 90% in a dominant right coronary system, competitive flow is a risk factor for arterial graft failure, and lesser lesions may be more safely grafted with a SV or left untouched. Moderate right coronary lesions (40-69%) have a lower rate of progression than often assumed and may reasonably remain ungrafted (14). The use of off-pump techniques favors arterial conduits, given several reports of poorer SVG patency after off-pump coronary artery bypass (OPCAB), and with anaortic OPCAB techniques being performed almost exclusively with multiple arterial grafts.
The management of isolated left main coronary artery stenosis presents an interesting problem. Should two grafts be used when the disease affects the origin or the body of the left main coronary artery where no stenosis exists between the major branches? Most surgeons graft the left anterior descending (LAD) and circumflex systems for treatment of bifurcation disease and more proximal lesions because of wide separation of the two territories. Will these grafts compete? Can this be balanced by equivalence of the grafts (bilateral in situ, ITAs), or by connecting the grafts as a Y-graft so that they arise from a single inflow as the LAD and circumflex do?
Graft harvest techniques
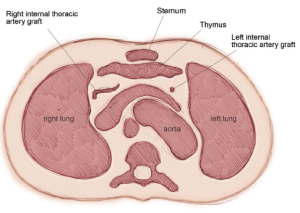
The ITAs are skeletonized and harvested extending from the inferior border of the subclavian vein distally to the bifurcation into the inferior epigastric and musculophrenic arteries. Extrapleural harvesting can be achieved with careful opening of the sternum and harvesting. This technique is beneficial in elderly patients and those with respiratory comorbidities by hastening recovery times. A disadvantage of protecting the pleural cavities is that it may restrict the access and ability to position the heart during OPCAB surgery.
RAs are removed by an open method with double clipping, cautery or ultrasound. Endoscopic radial harvesting is growing in popularity and non-randomized published data suggests this is a safe alternative. Pharmacologic dilatation is essential to prevent vasospasm in the perioperative period: glyceryl trinitrate, phosphodiesterase inhibitors or phenoxybenzamine may be employed, and each has its benefits.
Graft configurations
BITA grafting is desirable and forms the cornerstone of total arterial revascularization. The configuration for arterial grafting depends on a number of patient and anatomical variables. The greatest benefit may derive from grafting both ITA grafts to the left system, as is mandated in the largest randomized trial of single vs. bilateral ITA grafting.
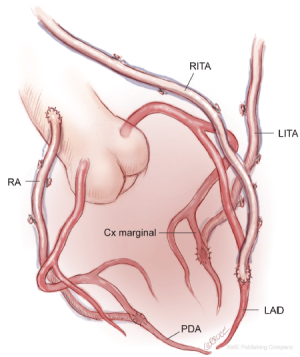
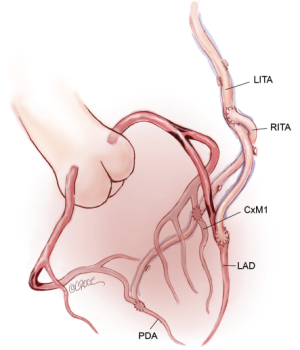
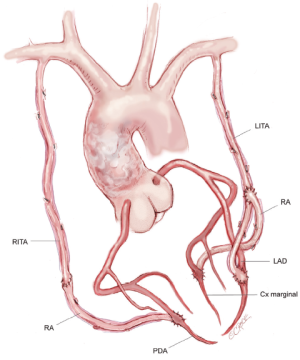
Use of bilateral in situ ITAs can be achieved in a number of different ways. One of the simplest methods, and our preferred technique, is to attach the in situ LITA graft to the circumflex or intermediate system on the left side either singly, sequentially to two lateral wall targets, or using a short segment of radial artery as a Y graft for the latter. The RITA is later anastomosed to the appropriate section of the LAD coronary artery, with or without a Y graft of radial artery to a diagonal branch. The arterial reconstruction is completed by grafting the RA to a branch of the right coronary system (Figure 1). Prior to grafting, the length of the right internal thoracic artery (RITA) is checked to ensure that it reaches the LAD target in a gentle curve above the aorta, and that it crosses the midline on the innominate vein behind the thymic fat, which provides safety for reoperation, as the RITA and LITA will be found close together as they enter the pericardium via a common slit.
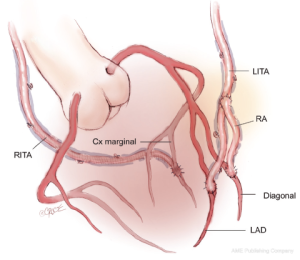
An alternative technique is to anastomose the LITA to the LAD, and if required, to a diagonal branch using either a sequential or Y-graft technique. Sequential grafting is only satisfactory when the diagonal branch lies adjacent to the LAD, thus avoiding a large loop of LITA which may angulate and thus compromise the distal anastomosis. A better technique is to use a short segment of additional arterial graft in a Y configuration from the LITA to the diagonal branch. This is a more flexible solution, allows for any diagonal location, and permits the LITA to find its own lie more easily without kinking when the chest is closed. With this strategy, the in situ RITA may be passed through the transverse sinus and anastomosed to the marginal branch of the circumflex system with the potential for a Y graft of radial artery off this to a second marginal target. Occasionally the RITA may be more easily brought anteriorly across the midline—behind the thymus as in the above description—to reach an intermediate or very proximal marginal branch. The reconstruction is then completed with a RA graft to the posterior descending branch (Figure 2). This technique is popular with some surgeons although it is difficult to visualize the entire length of the artery and any bleeding sites as it passes through the transverse sinus to the left system, and the posteriorly placed RITA may be difficult to control at reoperation.
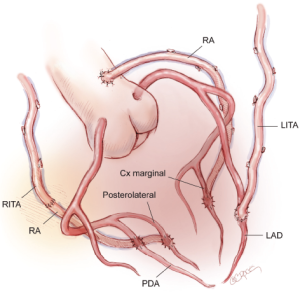
A third option is for the LITA to be grafted to the LAD and a RA graft to the circumflex system. The in situ RITA may be anastomosed to the main right coronary artery or to the terminal branches of the right coronary artery (RCA) using a graft extension technique. A RITA-RA graft extension may terminate in a single distal anastomosis or as a sequential graft with a side-to-side anastomosis to the posterior descending artery (PDA) and an end-to-side anastomosis with the posterolateral branch (Figure 3). In situ RITA grafts to the main RCA have suboptimal patency in both our experience and that of others, and this configuration, although technically simple and convenient, is not widely preferred.
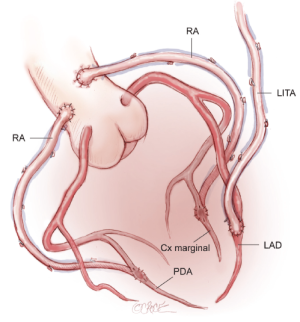
When BITAs are contraindicated, or in the very elderly, a total arterial reconstruction can be performed safely in almost all patients using the LITA to the LAD, supplemented by bilateral RA grafting to the circumflex system and to the RCA. This use of bilateral RAs is well tolerated in elderly patients in whom extensive SV graft disease is common. Avoiding the long SV avoids leg trauma and promotes early mobilization (Figure 4).
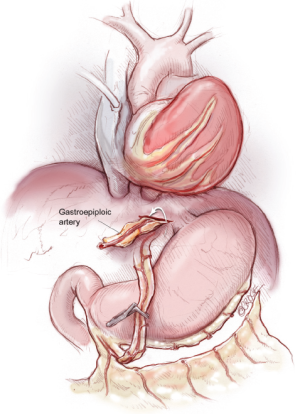
Management of extensive atheroma or calcification of the ascending thoracic aorta remains a challenge. Off-pump surgeons have pioneered the anaortic no-touch technique using single or bilateral ITA composite grafts (15-17) (Figure 5). A popular technique involves grafting the in situ LITA to the LAD and joining the free RITA or RA as a Y graft to the LITA for distal sequential anastomoses to the branches of the circumflex and RCAs. This has been used successfully by several authors (18,19). There is a potential risk of failure in using a single inflow although this is believed to have adequate flow reserve. There is also the potential for a steal phenomenon as well as a reduction in patency of the distal LITA-LAD segment has been reported, which is regarded as a major concern by our group. When anaortic OPCAB is undertaken, we prefer to use bilateral in situ ITAs to graft the left circulation, with a RA as Y graft from the circumflex graft to reach around the lateral wall to the posterior descending artery. Alternatively we have used an RA graft to the PDA from the aorta using the ingenious Heartstring device (Maquet Getinge Group, San Jose, California, USA) for a clampless proximal anastomosis on the aorta. The use of three in situ arterial conduits by addition of the right gastroepiploic graft to the posterior descending coronary artery is another more technically demanding option (11,20) (Figure 6).
Composite grafting
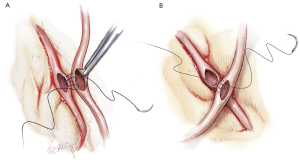
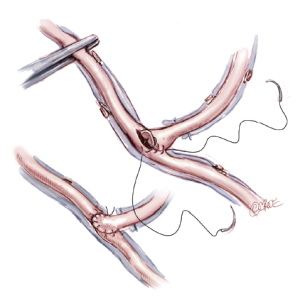
Sequential grafting (Figure 7A,B), composite Y-grafts (Figure 8) and extension grafts (Figure 9) are ancillary procedures allowing additional target artery anastomoses by the efficient use of an arterial conduit. They minimize peripheral incisions for conduit harvesting and may even allow six distal anastomoses in diffuse disease. The LITA-RITA Y-graft technique of Calafiore and Hwang allows complete revascularization based on two intra-thoracic conduits only (18,19) (Figure 10).
Comments
Arterial graft patency
Current patency data confirm that ITA grafts function into the third decade with freedom from failure in over 80%. Most of the later data relates to the widely recognized outstanding results from the LITAs. Tatoulis recently published results of a series of 991 right ITA grafts from 5,766 patients. There was no significant difference between the RITA and the LITA when grafted to the LAD (96.5% vs. 94.5%) and similar patencies between RITAs and LITAs were found when grafted to the circumflex system (90.5% and 88.5% respectively). When grafted to the RCA, the in situ RITA results were less satisfactory, but arterial grafts were far superior to SVGs (21). These data support the belief that the RITA behaves in a similar way to the LITA and that more effort should be made by surgeons to explore the potential benefits of the RITA.
There are a large number of observational studies but few randomized trials comparing the RA with the SVG. The 5-year results of the Radial Artery Patency Study (RAPS) confirm a functional benefit of the RA in comparison to the SVG. The Radial Artery Patency and Clinical Outcomes (RAPCO) trial is a two-tiered 10-year biological comparison of the RA vs. the free RITA or the SVG. No significant differences were found in survival or patency between the groups at 5 years.
Two large studies of approximately 1,000 gastroepiploic artery (GEA) conduits have indicated 5-year patencies of 62% and 86%. The GEA patency was similar to that of the SV (11,22). More recently, Suzuki reported that skeletonized GEAs had superior patencies to that of SVs (13).
Total arterial revascularization is achievable in most patients with three-vessel CAD (23). The major deterrent in this group of patients is the risk of sternal complications. This is found in patients with diabetes, obesity and pulmonary complications. With careful selection by avoiding these high-risk patients, complete arterial revascularization is readily achievable using skeletonized ITAs and yields good long-term results, even in patients with reduced ventricular function (24).
Clinical outcomes after arterial grafting
Grafting both ITAs to the left coronary system is recommended by most surgeons. Recently, the location of the second ITA was suggested not to influence the outcome of coronary bypass grafting (25). In a propensity score-matched analysis, Ruttmann compared two groups: the bilateral ITA-SVG and the LITA-RA-SVG group. The incidence of perioperative major adverse cardiac and cerebrovascular events was significantly lower in the RITA compared to the RA groups (1.4% vs. 7.6%, P<0.001). They concluded that this study provided strong evidence for the superiority of a RITA graft in comparison to a RA graft as a second conduit in multiple arterial revascularization (26).
Higher risk patients
Patients with diabetes mellitus merit special consideration as they are at an increased risk of sternal complications from the use of extensive arterial grafting, particularly from BITA grafting. In this subset of patients the risk of sternal complications has increased from less than 1% to about 3% (27-29). A fear of sternal complications is the main cause of surgeons rejecting the use of the RITA during a diabetic arterial reconstruction. An alternative technique is using skeletonization of the graft pedicle to reduce trauma to the chest wall and minimize sternal infections (30). In addition, the combination of single ITA grafts with one or both RA conduits may also reduce chest wall complications.
Total arterial grafting is readily applicable in many very elderly patients. Meticulous harvesting of the ITAs and preservation of the integrity of the pleural cavities reduces postoperative morbidity, particularly pulmonary status, and lowers hospital costs (31). We had adopted a similar extrapleural technique combined with skeletonization of the ITAs in 2001, which produced similar results in elderly patients (Figure 11). Most surgeons who practice extensive arterial grafting restrict BITA usage in the eighth decade (32-34). Compared with SV harvesting, a single ITA graft to the LAD, combined with RA conduits, is a safer alternative with a higher degree of patient satisfaction.
There are situations that preclude both BITA use and RA harvesting. In these contexts, and in moderate coronary lesions which risk competitive flow, the use of the SV is mandated. However, for the majority of patients with diffuse multivessel disease who dominate surgical caseloads in the post-percutaneous coronary intervention era, total arterial revascularization is achievable. This provides a durable and excellent clinical outcome with lower harvest site complications than from using the SV, as well as potential for lower progression of native vessel disease, higher graft patency and optimal long-term survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Konstantinov IE, Vasilii I. Kolesov: a surgeon to remember. Tex Heart Inst J 2004;31:349-58. [PubMed]
- Barner HB. Double internal mammary-coronary artery bypass. Arch Surg 1974;109:627-30. [PubMed]
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855-72. [PubMed]
- Tector AJ, Kress DC, Schmahl TM, et al. T-graft: a new method of coronary arterial revascularization. J Cardiovasc Surg (Torino) 1994;35:19-23. [PubMed]
- Tector AJ, Amundsen S, Schmahl TM, et al. Total revascularization with T grafts. Ann Thorac Surg 1994;57:33-8; discussion 39. [PubMed]
- Calafiore AM, Di Giammarco G, Luciani N, et al. Composite arterial conduits for a wider arterial myocardial revascularization. Ann Thorac Surg 1994;58:185-90. [PubMed]
- Dion R. Complete arterial revascularization with the internal thoracic arteries. Operative Tech Card Thorac Surg 1996;1:84-107.
- Kelly R, Buth KJ, Légaré JF. Bilateral internal thoracic artery grafting is superior to other forms of multiple arterial grafting in providing survival benefit after coronary bypass surgery. J Thorac Cardiovasc Surg 2012;144:1408-15. [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31:2470-81. [PubMed]
- Acar C, Ramsheyi A, Pagny JY, et al. The radial artery for coronary artery bypass grafting: clinical and angiographic results at five years. J Thorac Cardiovasc Surg 1998;116:981-9. [PubMed]
- Suma H, Tanabe H, Takahashi A, et al. Twenty years experience with the gastroepiploic artery graft for CABG. Circulation 2007;116:I188-91. [PubMed]
- Dimitrova KR, Hoffman DM, Geller CM, et al. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg 2012;94:475-81. [PubMed]
- Suzuki T, Asai T, Matsubayashi K, et al. In off-pump surgery, skeletonized gastroepiploic artery is superior to saphenous vein in patients with bilateral internal thoracic arterial grafts. Ann Thorac Surg 2011;91:1159-64. [PubMed]
- Hayward PA, Zhu YY, Nguyen TT, et al. Should all moderate coronary lesions be grafted during primary coronary bypass surgery? An analysis of progression of native vessel disease during a randomized trial of conduits. J Thorac Cardiovasc Surg 2013;145:140-8; discussion 148-9.[PubMed]
- Kim WS, Lee J, Lee YT, et al. Total arterial revascularization in triple-vessel disease with off-pump and aortic no-touch technique. Ann Thorac Surg 2008;86:1861-5. [PubMed]
- Yan TD, Vallely MP. Aortic no-touch off-pump coronary artery bypass grafting: a state-of-the-art surgical technique. Ann Thorac Surg 2010;90:1392-3. [PubMed]
- Ross DE. Anaortic coronary bypass surgery. Semin Thorac Cardiovasc Surg 2012;24:90-2. [PubMed]
- Calafiore AM, Contini M, Vitolla G, et al. Bilateral internal thoracic artery grafting: long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg 2000;120:990-6. [PubMed]
- Hwang HY, Kim JS, Cho KR, et al. Bilateral internal thoracic artery in situ versus y-composite graftings: five-year angiographic patency and long-term clinical outcomes. Ann Thorac Surg 2011;92:579-85; discussion 585-6. [PubMed]
- Glineur D, D’hoore W, El Khoury G, et al. Angiographic predictors of 6-month patency of bypass grafts implanted to the right coronary artery a prospective randomized comparison of gastroepiploic artery and saphenous vein grafts. J Am Coll Cardiol 2008;51:120-5. [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit--5,766 patients and 991 angiograms. Ann Thorac Surg 2011;92:9-15; discussion 15-7. [PubMed]
- Takahashi K, Daitoku K, Nakata S, et al. Early and mid-term outcome of anastomosis of gastroepiploic artery to left coronary artery. Ann Thorac Surg 2004;78:2033-6; discussion 2036. [PubMed]
- Itagaki S, Cavallaro P, Adams DH, et al. Bilateral internal mammary artery grafts, mortality and morbidity: an analysis of 1 526 360 coronary bypass operations. Heart 2013;99:849-53. [PubMed]
- Galbut DL, Kurlansky PA, Traad EA, et al. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: a propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg 2012;143:844-853.e4.
- Kurlansky PA, Traad EA, Dorman MJ, et al. Location of the second internal mammary artery graft does not influence outcome of coronary artery bypass grafting. Ann Thorac Surg 2011;91:1378-83; discussion 1383-4. [PubMed]
- Ruttmann E, Fischler N, Sakic A, et al. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation 2011;124:1321-9. [PubMed]
- Boodhwani M, Lam BK, Nathan HJ, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766-73. [PubMed]
- Toumpoulis IK, Theakos N, Dunning J. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg 2007;6:787-91. [PubMed]
- Cohen AJ, Lockman J, Lorberboym M, et al. Assessment of sternal vascularity with single photon emission computed tomography after harvesting of the internal thoracic artery. J Thorac Cardiovasc Surg 1999;118:496-502. [PubMed]
- Momin AU, Deshpande R, Potts J, et al. Incidence of sternal infection in diabetic patients undergoing bilateral internal thoracic artery grafting. Ann Thorac Surg 2005;80:1765-72; discussion 1772.
- Bonacchi M, Prifti E, Giunti G, et al. Respiratory dysfunction after coronary artery bypass grafting employing bilateral internal mammary arteries: the influence of intact pleura. Eur J Cardiothorac Surg 2001;19:827-33. [PubMed]
- Elmistekawy EM, Gawad N, Bourke M, et al. Is bilateral internal thoracic artery use safe in the elderly? J Card Surg 2012;27:1-5. [PubMed]
- Kieser TM, Lewin AM, Graham MM, et al. Outcomes associated with bilateral internal thoracic artery grafting: the importance of age. Ann Thorac Surg 2011;92:1269-75; discussion 1275-6. [PubMed]
- Calafiore AM, Di Mauro M, Di Giammarco G, et al. Single versus bilateral internal mammary artery for isolated first myocardial revascularization in multivessel disease: long-term clinical results in medically treated diabetic patients. Ann Thorac Surg 2005;80:888-95. [PubMed]





