Gastroepiploic artery graft in coronary artery bypass grafting
The right gastroepiploic artery (GEA) was used for indirect myocardial revascularization (Vineberg’s procedure) for the posterior or inferior wall of the heart in the late 1960s by Bailey et al. (1) and its angiographic patency was demonstrated in 1969 by Hirose et al. (2). With the development of coronary artery bypass grafting (CABG) procedures, direct anastomosis of GEA to the right coronary artery was attempted by Sterling Edwards (3) in early 1970s, but there was no exact documentation of the procedure.
In half a century of CABG, several kinds of conduits have been utilized and assessed. It is now clear that the saphenous vein graft deteriorates with time and the occlusion rate reaches up to 50% in 10 years after CABG, mainly due to atherosclerosis in the graft called “vein graft disease”. The internal thoracic artery (ITA) graft, on the contrary, stays patent in the long-term period and this evidence directly relates to the superior late outcomes in terms of longevity and postoperative cardiac events (4).
To extend the use of arterial conduits for myocardial revascularization, several autologous arteries have been investigated and utilized clinically. The GEA graft already has a 27-year history in CABG, and its clinical results are excellent, without an increase in perioperative risk (5-7). With proper use of multi-arterial conduits, CABG can be performed safely and with better long-term outcomes.
Anatomy
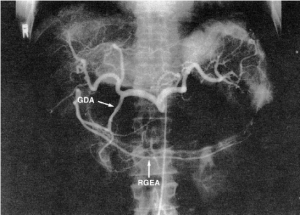
The GEA is the largest terminal branch of the gastroduodenal artery, the other branch being the superior pancreaticoduodenal artery. The gastroduodenal artery arises from the common hepatic artery in 75% of cases, and it may also branch away from the right or left hepatic artery, the accessory left hepatic artery, or the celiac trunk (Figures 1,2). In the rare case in which the gastroduodenal artery is absent, the GEA arises from the superior mesenteric artery (Figure 3). The GEA may run through an arcade formed by the gastroduodenal artery and the superior mesenteric artery. It runs between the posterior surface of the proximal region of the duodenum and the anterior surface of the pancreas head, along the lower margin of the pylorus, and then along the greater curvature of the stomach, together with the right gastroepiploic vein toward the left between two layers of the gastrocolic omentum.
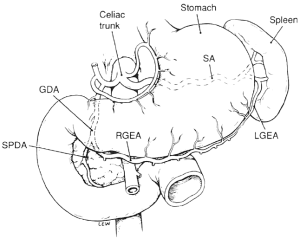
The GEA has been reported to reach two-thirds, one-half, and one-third of the greater curvature of the stomach in 34%, 61%, and 5% of cases, respectively (5). Thus, in many cases, the RGEA reaches more than half of the greater curvature. The diameter of the RGEA is 3 mm or more at its origin and is 1.5-2 mm in the middle of the greater curvature.
The mode of termination of the RGEA is variable. The GEA forms a continuous arcade with the left gastroepiploic artery in 35%, forms plexiform anastomoses in 15%, has no communication in 45%, and forms indirect anastomoses through the epiploic artery in 5% of cases.
Physiological and biochemical findings
Regarding responses to various drugs (8-10), the GEA shows contraction to ergonovine, serotonin, and phenylephrine similarly to the ITA. The GEA is more strongly contracted by potassium chloride, serotonin, and norepinephrine than the ITA, therefore it is important to prevent spasm of the GEA provoked by platelet aggregators, adrenergic stimulation, or depolarizing agents.
The GEA and the internal thoracic array have different responses to histamine (11). This agent causes contraction of the ITA but dilatation of the GEA. Toda et al. (12) have reported that the GEA at the proximal site is contracted by dopamine in a dose-dependent manner, but at the distal site it dilates at low concentrations and contracts at high concentrations. This finding indicates that the GEA contains mainly α-adrenoreceptors proximally and has both α-adrenoreceptors and α-dopaminergic receptors distally.
Our clinical investigation using an implantable ultrasonic Doppler miniprobe demonstrated that GEA blood flow is increased after a meal (13). This finding is in concordance with the above-mentioned response to histamine, that is, there may be an organ-specific reaction in which the GEA is dilated by histamine released after a meal to increase blood supply to the digestive tract. This biological response is clearly observed in the GEA graft connected to the coronary artery early after operation.
Mobilization of the GEA
Surgical procedure
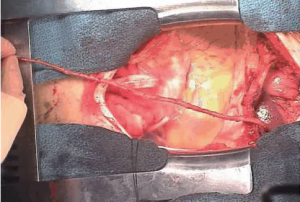
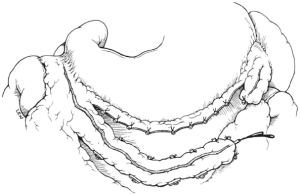
To harvest a GEA graft, the median sternotomy incision is extended to midway between the xiphoid process and the umbilicus, and the peritoneum is opened. The stomach is delivered into the wound, and the GEA is palpated along its greater curvature. It is important that the vessel is handled gently while evaluating its thickness, length and pulsatility, because manipulation readily induces spasm in the artery. The GEA and surrounding tissue is detached from the greater curvature as a pedicle (Figure 4). The branches to the omentum are usually small and easily divided by electrocautery. Branches to the stomach, however, should be ligated individually with a silk ligature or clip before they are divided because they are thick and short. If hemostasis at this site is not secure, a hematoma will develop in the pedicle. Mobilization of the GEA should begin at the lower margin of the pylorus and need not include the posterior aspect of the duodenum. Detachment then proceeds to include one-half to two-thirds of the greater curvature. The GEA pedicle should be made as long as possible, based on preservation of strong pulsation. Detachment of the GEA from the greater curvature, performed in the manner described, carries no risk of gastric ischemia (6).
The GEA is divided distally, and 3 to 4 mL papaverine hydrochloride (40 mg diluted in 10 mL of physiologic saline) is injected intraluminally to relieve spasm without clamping the graft proximally. The distal end is then clamped so that the artery is dilated by both the pharmacologic effect of the papaverine and distended by its own blood pressure. Leaving the proximal end unclamped during injection prevents intimal damage due to excessive hydrostatic pressure. With the distal end of the GEA clamped, the pedicle is checked for bleeding. The distance to the site of the anastomosis is measured to determine the length of the graft. The tissue surrounding the GEA adjacent to the site of anastomosis is ligated, and 2 to 3 cm of the artery is skeletonized. The right gastroepiploic vein running along with the right GEA is also ligated. The many direct communications between the artery and veins in the pedicle may lead to a significant amount of bleeding unless the vein is ligated securely. The distal end of the skeletonized GEA is transected, cut back a few millimeters in preparation for the anastomosis, and checked to ensure free flow (Figure 5).
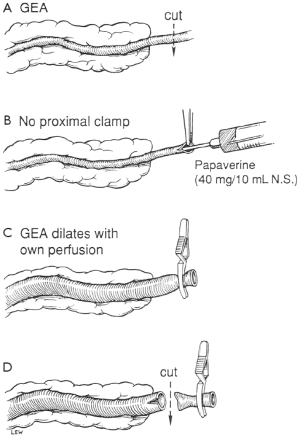
When making a totally skeletonized GEA graft, the skeletonized GEA (Figure 6) is harvested using the Harmonic Scalpel with a coagulating shears tip. The anterior layer of the greater omentum is divided and the GEA is exposed along its entire length. The small omental and gastric branches of the GEA are divided. The distal end of the GEA graft is then divided and clipped, and papaverine solution is infused intraluminally to relieve spasm of the GEA. The skeletonized GEA is then wrapped in a papaverine-soaked sponge. The papaverine preparation ensures that the GEA becomes a maximally dilated, relaxed arterial conduit. The skeletonized GEA is brought anteriorly to the pylorus and introduced into the pericardial cavity through the diaphragm.
Following institution of cardiopulmonary bypass an opening one- to two-fingers' breadth in diameter is made in the diaphragm using electrocautery and the GEA is delivered into the pericardial space avoiding any twisting of the pedicle. Only a simple incision in the anterior diaphragm is required for GEA grafting to the left anterior descending artery. The GEA pedicle can reach the pericardial space by traversing the anterior surface of the stomach and liver (Figure 7).

The GEA-coronary artery anastomosis is performed during cardioplegic arrest or off pump beating heart using a 7-0 or 8-0 polypropylene suture. The suture is placed in the heel of the graft using two to three stitches and then traction is applied to approximate the GEA to the coronary artery. The anastomosis is then completed using a single continuous suture. After three-quarters of the anastomosis has been carried out, a probe is introduced through the anastomosis to confirm patency and, if satisfactory, the rest of the suture line is completed (Figure 8).
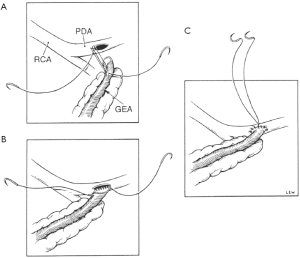
The direction of the anastomosis depends upon its site. When the target is the posterior descending or distal main right coronary artery, the GEA is anastomosed in an antegrade fashion, placing the heel of the graft on the proximal end of the arteriotomy. A retrograde anastomosis can be created by placing the heel of the graft on the distal aspect of the arteriotomy, when the target is the anterior descending or, rarely, the proximal right main coronary artery. I prefer an antegrade anastomosis for the circumflex artery.
Once the anastomosis has been completed the GEA is unclamped, and anastomotic haemostasis is checked before the aortic cross-clamp is released. The GEA pedicle is then fixed to the epicardium near the site of the anastomosis. At this time, great care must be taken to avoid kinking or twisting the skeletonized distal GEA in the area of the anastomosis (Figure 9). After the pedicle has been sutured to the epicardium, blood flow through the GEA frequently induces beating of the heart or coarse ventricular fibrillation. This is good evidence of satisfactory perfusion. Release of the aortic cross-clamp allows the heart to return to its normal size. The pedicle is inspected carefully to make sure that blood flow is unimpeded. The point of fixation should not distort the anatomy. Excess pedicle, if present, is pulled back into the abdominal cavity. The patient is weaned off cardiopulmonary bypass, and the operative fields are again checked for bleeding. Once haemostasis is secure, chest and abdominal wounds are closed. It is usually not necessary to place a drainage tube in the abdominal cavity.
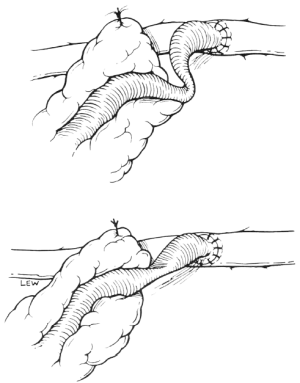
When the GEA is used as a free graft, preparation of the graft and anastomosis to the coronary artery are performed in the same manner as for in situ grafting. The only difference is that this requires a proximal anastomosis. The proximal anastomotic sites for the GEA include the ascending aorta, SV graft, or ITA graft. When the ascending aorta is thickened or has atherosclerotic lesions, direct anastomosis to the ascending aorta should be avoided.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Bailey CP, Hirose T, Brancato R, et al. Revascularization of the posterior (diaphragmatic) portion of the heart. Ann Thorac Surg 1966;2:791-805.
- Hirose T, Yaghmai M, Vera CA. Cineangiographic visualization technique of the implanted right gastroepiploic artery of the posterior myocardium. Vasc Surg 1969;3:61-7. [PubMed]
- Edwards WS, Lewis CE, Blakeley WR, et al. Coronary artery bypass with internal mammary and splenic artery grafts. Ann Thorac Surg 1973;15:35-40. [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [PubMed]
- Suma H, Fukumoto H, Takeuchi A. Coronary artery bypass grafting by utilizing in situ right gastroepiploic artery: basic study and clinical application. Ann Thorac Surg 1987;44:394-7. [PubMed]
- Suma H, Wanibuchi Y, Furuta S, et al. Does use of gastroepiploic artery graft increase surgical risk? J Thorac Cardiovasc Surg 1991;101:121-5. [PubMed]
- Suma H, Tanabe H, Takahashi A, et al. Twenty years experience with the gastroepiploic artery graft for CABG. Circulation 2007;116:I188-91. [PubMed]
- Koike R, Suma H, Kondo K, et al. Pharmacological response of internal mammary artery and gastroepiploic artery. Ann Thorac Surg 1990;50:384-6. [PubMed]
- Dignan RJ, Yeh T Jr, Dyke CM, et al. Reactivity of gastroepiploic and internal mammary arteries. Relevance to coronary artery bypass grafting. J Thorac Cardiovasc Surg 1992;103:116-22; discussion 122-3. [PubMed]
- O’Neil GS, Chester AH, Schyns CJ, et al. Vascular reactivity of human internal mammary and gastroepiploic arteries. Ann Thorac Surg 1991;52:1310-4. [PubMed]
- Ochiai M, Ohno M, Taguchi J, et al. Responses of human gastroepiploic arteries to vasoactive substances: comparison with responses of internal mammary arteries and saphenous veins. J Thorac Cardiovasc Surg 1992;104:453-8. [PubMed]
- Toda N, Okunishi H, Okamura T. Responses to dopamine of isolated human gastroepiploic arteries. Arch Int Pharmacodyn Ther 1989;297:86-97. [PubMed]
- Takayama T, Suma H, Wanibuchi Y, et al. Physiological and pharmacological responses of arterial graft flow after coronary artery bypass grafting measured with an implantable ultrasonic Doppler miniprobe. Circulation 1992;86:II217-23. [PubMed]