Bilateral internal thoracic artery grafting
The concept of using systemic arteries as grafts to coronary arteries began, as so many surgical concepts have, with experimental work by Alexis Carrel in 1910. Myocardial revascularization then came to fruition clinically with the Vineberg operation in the 1950s and early 1960s, during which one or both of the internal thoracic arteries (ITA) were implanted into the myocardium with the hope that connections would develop between ITA branches and small myocardial vessels, thereby increasing blood flow to the myocardium. The Vineberg operation was sometimes successful, but had inconsistent effectiveness and did not relieve ischemia acutely. The development of coronary angiography in the late 1950s led to an enhanced understanding of the obstructive patterns of coronary atherosclerosis and to the concept of bypass grafting, which involves creating a direct anastomosis between a graft and a coronary artery in a location distal to significant coronary stenoses. Experimental work was carried out utilizing a variety of bypass conduits including the ITA and saphenous vein grafts (SVG); but when the clinical coronary bypass grafting era began in 1967, the primary conduit used was the saphenous vein.
Throughout the early years of clinical coronary bypass grafting, ITA grafts were used in a few centers for direct anastomoses to the left anterior descending coronary artery (LAD), but the clinical use of this strategy did not become widespread, and was rarely used routinely even in centers where it was employed. The historical, theoretical and practical objections to ITA use, even as a single graft, are worth considering because some similar considerations are cited today as objections to extending the use of ITA grafts for bilateral (BITA) grafting:
- Preparation of ITA grafts is more time-consuming than preparation of vein grafts;
- ITA to coronary anastomoses tend to be smaller, more delicate anastomoses than SVG to coronary anastomoses and use of the ITA may make bypass grafting more difficult and time consuming. Specific technical challenges such as intramyocardial coronary vessels or heavily atherosclerotic coronary vessels may further increase operative challenges;
- The ITA is a smaller bypass conduit than a vein graft and the ITA may therefore have a lower maximum blood flow, creating the risk of hypoperfusion if grafted to a large and tightly obstructed coronary artery. In addition, spasm of an ITA graft might compromise flow;
- Atherosclerotic disease of the subclavian artery might diminish flow into an ITA graft;
- The use of the ITA as a graft compromises blood flow to the sternum and might increase the risk of sternal wound complications.
None of these concerns are entirely without merit and it is likely that they all play a role in slowing the pace of adoption of left internal thoracic artery (LITA)-LAD grafts in the early years of bypass surgery. A possible advantage of the ITA graft was that it would have better long term patency rates than SVGs, but in the early years of bypass surgery few comparative data existed and there was no uniform agreement regarding this principle.
By the late 1970s patency data began to accumulate and it became clear that not only was early SVG patency imperfect and inferior to early ITA patency, with the passage of time new pathologic changes developed in SVGs that progressively compromised long term patency rates. Pathologic examination of vein grafts removed either at autopsy or at reoperation revealed the distinct atherosclerotic changes, and sequential studies of SVGs showed that by the end of the first decade after operation, close to 50% of SVGs were either occluded or showed stenotic angiographic changes. Sequential studies of ITA-LAD grafts not only demonstrated superior early patency rates, but also very low rates of late stenosis, occlusion, pathologic changes and progression from patency to non-patency. Therefore, the early patency of LITA-LAD grafts became even more superior to SVG grafts with the passage of time (1).
The impact of these observations was to increase the use of the LITA as a graft to the LAD, particularly in centers where it was already employed. Concerns that arterial grafting might increase the complexity of operations began to be allayed by multiple factors, including: the increased effectiveness of cardiac surgical training in microsurgery, the increased experience of existing surgeons with coronary surgery as well as improved optics, instrumentation and myocardial protection. The improvements in and reliability of strategies for myocardial protection made the length of cross clamp time less important in the definition of outcomes, and increased the importance of the level of the surgical correction achieved during cross clamp. As surgeons became more comfortable with the operative aspects of ITA grafting and grew increasingly convinced of its efficacy, the LITA-LAD graft became more commonly used. However, the clinical importance of the LITA-LAD graft had not yet been proven.
In 1986, a study by Loop and colleagues documented a substantial improvement in clinical outcomes during the first decade after bypass surgery in patients receiving an ITA to LAD graft as compared to those that received SVG to LAD grafts (2). This study examined the revascularization strategy for the LAD coronary artery in both single vessel and multivessel situations, comparing patients who received a LITA-LAD graft and vein grafts to other vessels with patients whose operation was a vein graft only strategy. This non-randomized retrospective study showed that clinical events of death, reoperation and myocardial infarction were less common over a ten-year follow-up period of time when the ITA—instead of a saphenous vein—was used to graft the LAD. It underscored not only the superior patency of ITA to LAD grafts but also its clinical importance, and was the single study that contributed most to the standardization of LITA-LAD grafts for coronary revascularization (2). Today the LITA-LAD graft is considered a benchmark of quality, and has become one of the few cardiac surgical strategies to have ever superseded debate. It is worthwhile remembering, however, that it took 20 years for the superiority of the LITA-LAD graft to become apparent and a decade after that for it to reach its current level of use, which is greater than 90% in coronary bypass operations.
During the 1970s, the use of both internal mammary arteries during coronary revascularization operations (BITA grafting) was uncommon, and if this strategy was employed at all, it was usually in situations where other bypass grafts were not available. However, in the late 1970s when we reviewed the clinical outcomes of the BITA strategy, we found that this small group of patients experienced excellent clinical outcomes, despite a high degree of incomplete revascularization and other relatively unfavorable patient-related characteristics (3).
These positive observations increased the use of the BITA strategy for elective coronary revascularization, and during the 1980s and early 1990s we began to accumulate an increasing number of patients for whom we had elected to expand the use of BITA grafts. A minority of other centers followed similar strategies, often because of a fundamental belief in the concept, and in doing so created patient subsets that have been subsequently available for clinical follow-up (4-7).
The BITA strategy was even slower to be adopted than the LITA-LAD. There are multiple reasons behind this, including that the BITA operation is more technically demanding, takes longer to do, and makes a simple operation more complicated. The most valid concern however, is that BITA grafting appears to increase the risk of wound complications. In addition, the incremental clinical value of BITA grafting was not clear and if it did exist, which patients benefited?
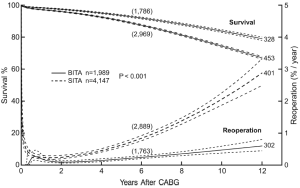
By the mid-1990s enough patients had undergone BITA grafting at our institution and had been followed long enough that we could submit some of these questions to study. We reviewed 2001 patients receiving BITA grafting and more than 8,000 receiving SITA grafting. This was a retrospective study, treatment was not randomized, and multiple statistical methods were used to address the issues of patient selection. All of these patients had undergone single ITA grafts to single coronary vessels usually as in situ grafts but sometimes as free (aorta to coronary) grafts. Sequential ITA grafting was rare. Many patients received SVGs in addition to ITA grafts and there were no patients who received composite ITA grafts. More than 1,000 patients in both groups were followed for more than ten post-operative years. Results of these analyses indicated that death, re-operation and percutaneous transluminal coronary angioplasty were more frequent in the follow-up period for patients who underwent SITA grafting when compared with those who received BITA grafting (7) (Figure 1). Although the results from similar studies were mixed and not all centers found an improved survival rate, in no situation was the survival rate worse with BITA grafting. At this point, few patients had been followed for more than ten post-operative years.
Our second study of these same patients was published in 2004 and added substantial follow-up data (8). This second study examined survival in detail, focusing on the issue of whether or not the apparent survival advantage for BITA grafting seen at twelve post-operative years in our initial study continued to be maintained at twenty post-operative years. Further analyses attempted to predict the magnitude of that survival advantage and whether or not the survival advantage was true for all patient subsets.
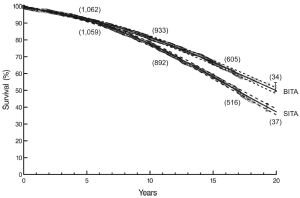
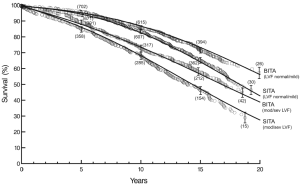
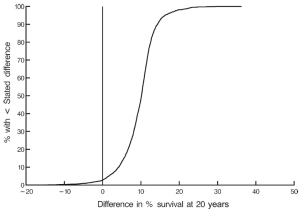
At twenty post-operative years BITA grafting improved the survival rate for the overall group (Figure 2) and for most patient subsets. For example, survival curves based on left ventricular function are shown in Figure 3. There was a small group of patients defined by advanced age and a small body surface area for which BITA grafting appeared to have worse long-term survival. The decreased survival rate for that subset was not of large magnitude and occurred early during the follow-up. Furthermore, the magnitude of the increased survival benefit for the majority of the patient subsets was predicted to be greater than 10% at twenty post-operative years (Figure 4). Thus, there was a general survival benefit associated with BITA grafting that extended for twenty post-operative years, which became increasingly apparent during the second post-operative decade. Over time, studies from other institutions have appeared to confirm these findings, particularly as the follow-up intervals increased. Thus, multiple data sets appear to show that the surgical strategy of BITA grafting incrementally improves the long-term survival rate (4,6,8,9).
Despite these data, a strong trend in the application of BITA grafting has not become evident. One reason is the issue of wound complications. Simultaneous use of both ITAs for bypass grafting does increase the risk of wound complications. Historically, this increase in risk was substantial, particularly for diabetic patients. Although risks have decreased today, even in a recent randomized trial of SITA versus BITA grafting, wound complications were slightly higher in the BITA group (10). Skeletonizing the ITA conduits, however, does appear to diminish sternal blood supply to a lesser degree than when the ITAs are prepared as pedicles, and clinical data seem to show that skeletonizing the ITAs ameliorates much of the increased risk of wound complications (11-13). This observation is a very important one and is a very substantial step forward, as even small numbers of sternal complications constitute a major disincentive to the use of BITA grafting. On the other hand, skeletonizing the ITAs make what is already a meticulous and time-consuming operation even more so.
There has also been concerns about whether or not the patency rates of ITA grafts to non-LAD vessels are as good as that for LITA-LAD. Although few modern prospective and inclusive ITA data exist, the data we do have available would indicate that ITA patency to circumflex and right coronary vessels is not as good as it is when the LAD is grafted. This observation also applies to all other bypass conduits, arterial or otherwise. However, the factor that appears to most negatively impact ITA patency is the existence of a high level of competitive coronary flow based on the presence of a non-significant stenosis in the grafted coronary artery. This is an observation that is also true of other arterial grafts. Within that context, it does not appear to make much of a difference whether the circumflex or the right coronary artery is involved and patency to both is not quite as good as patency to the LAD. However, the right ITA patency to those vessels appears to be better than other conduits as long as there is significant stenosis in the coronary artery (14,15).
It must be kept in mind that coronary surgery is a rapidly evolving process when considering long-term results. The BITA operations which have produced these findings have changed, even as these results have become more compelling today. The environment in which coronary surgery is performed has also changed.
Statin therapies as well as other concepts of risk factor management have appeared to make vein graft atherosclerosis a less widespread and aggressive process than that we have observed in the past, and may increase long-term SVG patency rates. Even if this is true, we do not yet have data that indicates that this is consistent over the long-term (>10 years) or that the patency of SVG can approach that of ITA grafts.
The use of the radial artery as a coronary bypass conduit has been revived and early favorable outcomes have raised the question of whether or not the radial artery is a satisfactory substitute for the right ITA. This is an attractive concept as the radial artery is a larger vessel, more forgiving to work with, and can be prepared at the same time as the left ITA is being prepared for bypass grafting. The RAPS trial and a number of clinical studies have shown that radial artery grafts does appear to have a patency advantage relative to SVG at five post-operative years (16,17). These analyses are still well within the first decade of follow-up and the long-term outcome is unknown. So far, no data indicate that the radial artery is superior to the right ITA or that its use produces better clinical outcomes; in fact, data that do exist suggest otherwise (18).
Off-pump surgery, a concept undertaken in an attempt to decrease the morbidity associated with coronary bypass surgery, has probably also served to negatively influence the use of multiple ITA grafts. Bilateral ITA grafting, particularly if sequential ITA grafts are employed, is a meticulous operation and is more difficult to perform without cardiopulmonary bypass, especially if additional technical challenges such as intra-myocardial or heavily atherosclerotic coronary arteries are present. The desire to perform off pump surgery therefore probably lessens the likelihood that complex arterial grafting will be employed.
A major technical change in BITA grafting has been the increased use of composite ITA grafts or “Y” ITA grafts. In this type of operation the right ITA is anastomosed to the left ITA and the right ITA is used to graft lateral and/or posterior wall vessels. This concept was used years ago by Sauvage et al. and further popularized by the work of Tecter and colleagues (19,20). We began to adopt this strategy in the early 1990s and at the present time it is the grafting pattern we use most commonly. The operation is based on an in situ left ITA graft. The right ITA is taken down as a free graft and is anastomosed to the left ITA prior to cardio-pulmonary bypass. The left ITA is then used to graft the left anterior descending and the right ITA is used to graft the vessels of the lateral wall and posterior vessels either as a sequential graft or as an isolated graft to the single most important vessel with vein grafts being used to other vessels. A relatively short length of the right ITA can be used to graft the circumflex vessels, although if the right coronary is also to be grafted with the right ITA the full length is required. This type of composite graft tends to remove the issue of whether or not there is sufficient length of the ITA to graft posterior vessels. Another major advantage of this strategy is that it avoids crossing the right ITA beneath the sternum to graft the LAD coronary artery. One of the hopes in the early days of bilateral ITA grafting was that it would eliminate the need for reoperations. That may be close to true in regard to coronary revascularization, but it is not true in regard to the development of other cardiac pathologies—such as aortic valve or ascending aorta pathology—during the decades that many BITA patients survive. Extensive studies of the patency of ITA composite grafts do not exist but the data that do exist lead us to believe that the same patency principles that had been shown to be true for single ITA grafts are also true for composite ITA grafting.
What then do we now know about BITA grafting? BITA grafting achieves better survival rates when compared with the single ITA strategy. Multiple data sets confirm this observation. Nonetheless, relatively few patients in America, even those who are good anatomic and clinical candidates for BITA grafting, receive that operation. Why? Today there is substantial institutional, professional and payer scrutiny about what is termed “quality”, and quality metrics are almost exclusively based on short-term outcomes and short-term processes. Thus, cardiac surgeons are greatly incentivized to function extremely well in regard to short-term outcomes but do not practice the same scrutiny regarding long-term outcomes. The only advantages of a complex ITA grafting operation are improved long-term outcomes. There does not appear to be a major short-term benefit and even if short-term patient outcomes are equivalent to other surgical strategies, BITA grafting usually involves increased operating time and increased use of resources, and is hence looked upon unfavorably in an era where efficiency and cost reduction are emphasized.
The biggest disadvantage hindering the extension of BITA grafting is that it is not a generic operation. Although it is the best that we can do for most patients, it is an operation that has not been generalized because it is not possible to generalize it. The level of skill, experience and concentration on the BITA technique are greater than that required for a standard bypass surgery operation. In this way, it is similar to operations such as the repair of thoraco-abdominal aneurysms, neonatal cardiac surgery, pulmonary valve auto-transplantation, valve-sparing aortic root operations and mitral valve repair. Few of us believe that all cardiac surgeons are equally skilled and experienced in performing all of these operations. The same is true of complex arterial grafting. It is a specialized operation that in the best of hands produces outcomes that, over the long-term, are superior to other strategies. However, technical imperfection carries with it substantial penalty. Bilateral grafting is not easy to teach, and as the number of bypass operations done in America has decreased it has become even more difficult to teach and for a surgeon to acquire the personal experience through repetition that is necessary for expertise. The situation is further complicated by the fact that the complex operations listed above do not form the fundamental basis of the practice of cardiac surgery at most institutions whereas coronary bypass surgery does. Thus, the concept of “send them someplace where they do a lot of these”, which tends to be true of many other complex operations, meets more resistance in regard to a coronary bypass operation.
Of course, the benefits of BITA grafting is not universally applicable and hence this technique is not indicated for every patient. Patients with a life expectancy of less than a decade or with multiple co-morbidities may not derive much benefit and there are anatomic situations where operating may be particularly difficult with increased risks. Those considerations are real but do not constitute good enough reasons not to perform BITA grafting in patients who are good candidates for it. BITA grafting is the best operation we can do for coronary revascularization over the long term. Today it is not tenable for a surgeon to ignore this concept and still be considered a serious coronary surgeon.
AcknowledgementsOther Section
Disclosure: The author declares no conflict of interest.
ReferencesOther Section
- Lytle BW, Loop FD, Cosgrove DM, et al. Long-term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J Thorac Cardiovasc Surg 1985;89:248-58. [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [PubMed]
- Lytle BW, Cosgrove DM, Saltus GL, et al. Multivessel coronary revascularization without saphenous vein: long-term results of bilateral internal mammary artery grafting. Ann Thorac Surg 1983;36:540-7. [PubMed]
- Buxton BF, Komeda M, Fuller JA, et al. Bilateral internal thoracic artery grafting may improve outcome of coronary artery surgery. Risk-adjusted survival. Circulation 1998;98:II1-6. [PubMed]
- Dion R, Glineur D, Derouck D, et al. Long-term clinical and angiographic follow-up of sequential internal thoracic artery grafting. Eur J Cardiothorac Surg 2000;17:407-14. [PubMed]
- Kurlansky PA, Traad EA, Dorman MJ, et al. Thirty-year follow-up defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg 2010;90:101-8. [PubMed]
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855-72. [PubMed]
- Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg 2004;78:2005-12; discussion 2012-4.
- Endo M, Nishida H, Tomizawa Y, et al. Benefit of bilateral over single internal mammary artery grafts for multiple coronary artery bypass grafting. Circulation 2001;104:2164-70. [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31:2470-81. [PubMed]
- Kamiya H, Akhyari P, Martens A, et al. Sternal microcirculation after skeletonized versus pedicled harvesting of the internal thoracic artery: a randomized study. J Thorac Cardiovasc Surg 2008;135:32-7. [PubMed]
- Matsa M, Paz Y, Gurevitch J, et al. Bilateral skeletonized internal thoracic artery grafts in patients with diabetes mellitus. J Thorac Cardiovasc Surg 2001;121:668-74. [PubMed]
- Galbut DL, Kurlansky PA, Traad EA, et al. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: a propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg 2012;143:844-53.e4.
- Sabik JF 3rd, Lytle BW, Blackstone EH, et al. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg 2003;76:1490-6; discussion 1497. [PubMed]
- Sabik JF 3rd, Lytle BW, Blackstone EH, et al. Comparison of Saphenous Vein and Internal Thoracic Artery Graft Patency by Coronary System. Ann Thorac Surg 2005;79:544-51; discussion 544-51. [PubMed]
- Deb S, Cohen EA, Singh SK, et al. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol 2012;60:28-35. [PubMed]
- Schwann TA, Engoren M, Bonnell M, et al. Comparison of late coronary artery bypass graft survival effects of radial artery versus saphenous vein grafting in male and female patients. Ann Thorac Surg 2012;94:1485-91. [PubMed]
- Ruttmann E, Fischler N, Sakic A, et al. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation 2011;124:1321-9. [PubMed]
- Sauvage LR, Rosenfeld JG, Roby PV, et al. Internal thoracic artery grafts for the entire heart at a mean of 12 years. Ann Thorac Surg 2003;75:501-4. [PubMed]
- Tector AJ, McDonald ML, Kress DC, et al. Purely internal thoracic artery grafts: outcomes. Ann Thorac Surg 2001;72:450-5. [PubMed]





