Twenty-year fate of the radial artery graft
In a recently published study, angiographic data collected over a twenty-year period of experience with the radial artery (RA) graft were reviewed (1). Between 1989 and 2009, 548 angiograms were performed in 351 patients with one or more radial grafts, representing 43% of the total cohort. While routine control angiograms using the conventional method were common practice in the early period, guidelines have changed our policy and more recently this technique was restricted to patients with evidence of myocardial ischemia, with a total of 343 conventional angiographies. Being less invasive, CT scan angiography was more readily prescribed in asymptomatic and elderly patients. It proved to be a good screening tool for assessing graft patency and this method was applied to assess long-term RA patency in 205 patients. A total of 1,427 coronary bypass grafts were opacified, including 629 radial artery grafts. The follow-up period extended for up to 20 years, which is currently the longest in the literature. Mean follow-up was 7.0 years.
RA graft stenosis
Focal stenosis of an otherwise normal radial artery graft occurred in 3% of all RA grafts at 20 years. Stenosis was sometimes present at an anastomosis, suggesting either a faulty surgical technique or intimal hyperplasia. More often, stenosis was located at the body of the RA graft, raising difficult pathophysiological issues (2). When detected early in the postoperative period, it was difficult to formally exclude a spasm refractory to in situ vasodilators and the stenosis was occasionally treated by balloon dilatation without stenting (3). If detected late, the stenosis was usually organic in nature. Some sites of narrowing possibly pre-existed prior to surgery, such as atheromatous plaque involving the radial artery at the forearm that had been overlooked at the time of surgery. Alternatively it could be related to fibrosis secondary to arterial trauma due to either poor harvesting technique or to previous RA catheterization such as in transradial coronary angiography, which has been shown to frequently induce intimal disruption and/or medial dissection. In some cases, RA stenosis definitely occurred secondarily, as clearly illustrated by a few instances in which the graft was found to be perfectly intact on previous angiogram imaging (2). It is not unlikely that these grafts had been the site of minor parietal injury that ultimately evolved towards a hemodynamic stenosis. All RA stenoses could safely be treated by direct angioplasty of the RA conduit and stenting offered a durable result (2). In contrast, percutaneous treatments for vein graft disease continue to be hindered by a high peri-procedural morbidity resulting from distal embolization of atherothrombotic debris. More importantly, the risk of detecting late RA graft stenosis could probably be eliminated by using strict preoperative criteria prior to harvesting, such as systematic preoperative echo-Doppler screening and exclusion of all calcified RAs as well as all previously catheterized conduits.
String sign
String sign was defined as a diffuse narrowing of the whole graft, unresponsive to in situ vasodilators. It was observed in 0.9% of all RA grafts. Graft involution was likely to result from competitive flow, which usually manifests early in the postoperative period (3). Reversal of the RA string sign has not been observed in our series; these grafts were non-functional and were classified as occluded.
Graft patency: comparison of RA and other conduits
At 7.0 years mean follow-up, the patency of the RA graft was 83% (1). Being retrospective and non-randomized, the present study did not permit a fair comparison between grafts. In this series, RA graft patency was significantly lower than that of the left IMA (96%, P<0.001) but it was not statistically different from that of the other grafts, including the right IMA (88%, P=0.32), free IMA (80%, P=0.60) and vein (82%, P=0.77). The variable extent of the target territory among grafts represented a serious bias. The superior patency of the left IMA grafts, which were almost always anastomosed to the LAD as opposed to the radial grafts (99% of non-LAD targets), could hardly be assigned solely to the type of conduit. The patency of the venous conduits was surprisingly high in this series and not significantly different from that of the radial grafts. With two-thirds of the patients undergoing all-arterial revascularization, the number of venous bypasses was small. When used, the venous segment was short, harvested at the leg and carefully selected. Only veins of faultless quality without varicosis were used. No attempt was made to revascularize small sized coronary branches, which would have increased the need for multiple venous conduits (the mean number of grafts was 2.45 graft/patient), as well as the risk of graft occlusion. For all these reasons, the patency rate of vein grafts in our study did not reflect what has generally been reported in the literature.
Determinants of RA graft patency
The high rate of opacification allowed identification of some determinants of radial artery graft patency (1). The incidence of graft occlusion approximately doubled when the indication for angiography was based on clinical evidence of myocardial ischemia. Since most angiographic studies were directed by symptoms, the exact RA graft patency was somewhat underestimated.
The patency of the radial artery conduits was directly influenced by the site of the target coronary. In our experience, the number of RA grafts anastomosed to the LAD was too small to allow proper statistical analysis in this territory. For the other targets, the 7-year patency of RA grafts decreased from 93% for the diagonal/intermediate artery, to 83% and 78% for the obtuse marginal and the right coronary artery respectively (P<0.01 and P<0.001 respectively).
Competitive flow has been recognized as one of the main causes of RA graft failure. Graft patency for targets with moderate stenosis was worse than that for vessels with critical stenosis. An original finding in our series was that surgical coronary revascularization in conjunction with valve surgery offered lower radial graft patency as compared to coronary bypass alone (P=0.05). When combined with valve surgery, the indication for coronary bypass often relied on the detection of coronary stenoses by routine preoperative angiography rather than on clinical evidence of myocardial ischemia. The risk of grafting a coronary artery with low-grade stenosis resulting in competitive flow was then higher and RA graft occlusion rate was increased. In addition to the aforementioned situation, competitive flow was encountered in cases of proximal left main stenosis whenever a patent left IMA-to-LAD graft provided unrestricted flow to the circumflex territory or in cases of chronic coronary occlusion whose distal run-off was supplied by an abundant collateral circulation.
On the whole, sequential or Y RA conduits had an increased patency compared to grafts with a single anastomosis, although the difference did not reach significance (91% versus 82% at 7 years, P=0.08). Occasionally, the proximal or the distal end of the graft remained patent whereas the rest of the graft was non-functional (i.e., string/occlusion).
Neither antianginal nor antithrombotic medications individually influenced RA graft patency. The prescription of calcium channel blockers was not identified as a determinant of radial artery patency. The use of an antispasmodic medication should no longer be a prerequisite to radial artery grafting.
RA graft patency over time
In order to analyze RA patency over time, 629 radial graft angiographies were separated into four equal groups (n=137) at successive follow-up intervals: 1.0, 5.4, 8.3 and 13.1 years (1). Radial artery graft patency tended to be higher in the first quartile: 86%, 82%, 81% and 82% respectively (P=0.2). Further subdivision in the first quartile showed that radial artery patency decreased significantly during the first postoperative year (93% versus 79% before and after 7 months respectively, P<0.01) and then remained stable throughout the whole twenty-year follow-up (1).
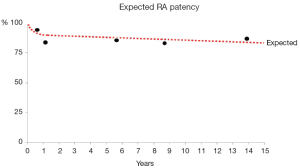
Analysis of recurrent opacifications of the same conduits was carried out in 159 RA grafts (up to 5 serial exams). All angiograms less than 6 months were compared with the closest second angiogram available so as to emphasize patency in the early phase. The late phase was assessed by comparing the first angiogram beyond 6 months with the latest control. This confirmed that the RA patency exhibited a rapid exponential decline within the first postoperative year. We believe that, aside from technical error, competitive flow was the main mechanism of early graft failure (3). Interestingly, beyond one year, radial artery graft patency was linear with a very low attrition rate (0.37% per year) for up to 20 years (Figure 1). This suggests a lack of radial graft disease in contrast to what is commonly observed with vein grafts. This has been confirmed in a recent report from our group in which CT angiography performed late after surgery showed a complete absence of radial artery wall calcification as opposed to occasional venous conduits whose walls were clustered with calcareous deposits (4).
Conclusions
The radial artery conduit has been observed to remain virtually free from graft disease over a 20-year period. Citing the seminal article by Carpentier published forty years ago in the Annals of Thoracic Surgery: “The aorta-to-coronary radial bypass graft” appears to be “a technique avoiding pathological changes in grafts” (5).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Achouh P, Isselmou KO, Boutekadjirt R, et al. Reappraisal of a 20-year experience with the radial artery as a conduit for coronary bypass grafting. Eur J Cardiothorac Surg 2012;41:87-92. [PubMed]
- Goube P, Hammoudi N, Pagny JY, et al. Radial artery graft stenosis treated by percutaneous intervention. Eur J Cardiothorac Surg 2010;37:697-703. [PubMed]
- Acar C, Jebara VA, Portoghese M, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg 1992;54:652-9; discussion 659-60. [PubMed]
- Achouh P, Boutekadjirt R, Toledano D, et al. Long-term (5- to 20-year) patency of the radial artery for coronary bypass grafting. J Thorac Cardiovasc Surg 2010;140:73-9, 79.e1-2.
- Carpentier A, Guermonprez JL, Deloche A, et al. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann Thorac Surg 1973;16:111-21. [PubMed]





