Aortic arch surgery using moderate hypothermia and unilateral selective antegrade cerebral perfusion
Introduction
Surgical treatment of aortic arch pathology requires partial or complete replacement of the aortic arch while the systemic circulation is temporarily interrupted. Patients undergoing this obligatory period of circulatory arrest are at an increased risk for adverse neurologic outcomes and ischemic end-organ damage. Therefore, strategies for cerebral protection and circulation management must be implemented to achieve optimal clinical results.
Since Griepp’s initial successful series of total arch replacements using deep hypothermic circulatory arrest (DHCA) alone, cerebral protection strategies and surgical techniques have evolved to produce improved clinical outcomes (1). Over the past two decades, multiple large series of arch replacements, with limited morbidity and mortality, have been reported in the literature using antegrade or retrograde cerebral perfusion during the period of hypothermic circulatory arrest (2-7). Cerebral protection strategies have continued to evolve with recent series reporting successful arch replacements conducted under moderate levels of hypothermia in conjunction with antegrade cerebral perfusion (8-11).
The optimal strategy of cerebral protection and circulation management during arch replacement has yet to be determined. In 2004 at Emory University Hospital, we instituted a protocol of hypothermic circulatory arrest and unilateral selective antegrade cerebral perfusion (uSACP)via the right axillary artery for all cases requiring aortic arch replacement. Initially we used deep hypothermia (18-22 °C) and achieved acceptable outcomes. However, in an attempt to limit cardiopulmonary bypass times and ameliorate the adverse effects of profound hypothermia we gradually transitioned to more moderate (>22 °C) levels of hypothermia (MHCA) at the initiation of circulatory arrest. The purpose of this report is to analyze the safety of MHCA and uSACP for hemi- and total arch replacement in both the elective and emergent settings.
Methods
This study was conducted under a protocol approved by the Institutional Review Board at the Emory University School of Medicine in compliance with HIPAA regulations and the Declaration of Helsinki. The Institutional Review Board waived the need for individual patient consent. A retrospective review of the Emory Aortic Surgery Database identified 733 patients who underwent aortic arch surgery using uSACP via cannulation of the right axillary artery and hypothermic circulatory arrest at the three primary hospitals within the Emory Healthcare system between January 2004 and December 2012. Patients were divided into groups based upon the extent of arch reconstruction (hemi-arch vs. total arch) and the indication for aortic replacement (aneurysm vs. acute type A aortic dissection). These divisions produced Elective (aneurysmal disease) and Emergent (acute type A aortic dissection) groups for outcomes analysis. Medical records were reviewed for pre-, intra-, and post-operative variables. Definitions were according to the Society of Thoracic Surgery National Database specification (http://www.sts.org/sites/default/files/documents/word/STSAdultCVDataSpecificationsV2_73%20with%20correction.pdf).
Operative technique
Anesthetic induction was achieved with standard techniques including administration of sodium pentothal, isoflurane, fentanyl, and muscle relaxant. A Foley catheter with a temperature probe was inserted to measure bladder temperature, which was used as the primary indicator of core body temperature. Transcutaneous cerebral oximetry (INVOS 3100-SD; Troy, Mich) and electroencephalogram monitoring were routinely performed in all cases.
All operations were initiated by exposing the right axillary artery with a deltopectoral groove incision. After administration of 5,000 U of heparin, a side-biting clamp was placed across the axillary artery. An 8-mm Gelweave graft (Vascutek; Terumo, Ann Arbor, Mich) was sewn end-to-side to the axillary artery using a running 6-0 polypropylene suture. The graft was cannulated with a 22 Fr Elongated Arterial Cannula (Medtronic Corp, Minneapolis, Minn) and attached to the cardiopulmonary bypass circuit. An additional arterial line was attached to a side port to measure cerebral perfusion pressures during the circulatory arrest period.
All patients were approached via median sternotomy incision. In cases performed for the treatment of aneurysmal disease, a cross-clamp was placed across the ascending aorta and myocardial arrest was achieved with cold blood cardioplegia. In cases performed for the treatment of aortic dissection, the aorta was not clamped unless the amount of aortic insufficiency overwhelmed the capacity of the left ventricular vent to adequately decompress the ventricle.
The goal core body temperature was variable and depended upon several factors including age, preoperative renal function, aortic pathology and the extent (hemi- vs. total arch) and complexity of the planned aortic arch reconstruction. After the initiation of cardiopulmonary bypass, the innominate artery was isolated proximal to the origin of the right subclavian artery. Upon reaching the goal core body temperature, the innominate artery was clamped proximally, and arterial inflow via the right axillary artery was lowered to 10 mL/kg/min. This provided unilateral antegrade cerebral perfusion at 16 °C via the right common carotid artery, and was adjusted to maintain cerebral perfusion pressures of 70-80 mmHg. In cases where cerebral oximetry saturations dropped ≥10% below baseline values, particularly on the left side, the left common carotid artery was isolated and clamped.
After initiation of the circulatory arrest period, all aortic arch pathology was resected. The open arch was routinely inspected for the presence of retrograde flow coming from the ostia of the left common carotid and subclavian arteries to verify the presence of antegrade cerebral perfusion via the right axillary artery. In all cases of hemi-arch replacement (HARCH), distal aortic reconstruction was performed with an open beveled anastomosis to the undersurface of the aortic arch. Once the arch reconstruction was complete, the aortic graft was clamped proximally and the clamp on the innominate artery was released, ending the circulatory arrest period. Full cardiopulmonary bypass was resumed immediately, restoring blood flow to the lower body.
Total arch replacement (TARCH) was defined as individual reimplantation of the brachiocephalic vessels, and performed using a 4-branch modified arch Gelweave™ graft. In these cases, the circulatory arrest period would end following completion of the distal aortic and left subclavian anastomoses. At this point, full cardiopulmonary bypass was reinstituted by cannulation of a sidebranch of the arch graft with a separate arterial cannula, and rewarming was initiated. Next the left common carotid artery anastomosis was performed, followed by proximal aortic reconstruction. The cross-clamp was removed prior to anastomosis of the innominate artery.
Statistical analysis
Operative mortality included in-hospital and 30-day mortality. Stroke or permanent neurologic dysfunction (PND) was defined as a new and permanent focal neurologic deficit with or without evidence of cerebral infarction on computed tomography or magnetic resonance imaging and was confirmed by a neurologist. Temporary neurologic dysfunction (TND) was defined as postoperative confusion, delirium, obtundation, or transient focal deficits (resolution within 24 hours) with negative brain computed tomography or magnetic resonance imaging scans (12). In this analysis, patients who died in the operating room or within 24 hours after surgery were excluded from the analyses of postoperative PND or TND only if neurologic assessment in these patients was not possible. All identifiable patients who died from complications of PND or TND were included.
Clinical outcomes were determined using t-tests and chi-square tests. To determine the independent effect of temperature, a multivariable logistic regression model was fitted without regard to issues of confounding or multicolinearity. This model sought to determine the effect of temperature adjusted for 4 major endpoints: mortality, PND, TND and renal failure requiring new postoperative dialysis. Adjusted odds ratios (AOR) along with 95% confidence intervals were computed to determine the magnitude of effect for a unit change in each of the predictors.
The data were analyzed with SAS Version 9.2 (Cary, NC). All statistical tests were evaluated at the 0.05 alpha level. All comparisons and model terms were preplanned.
Results
Hemi-arch replacement
500 patients underwent HARCH under MHCA with uSACP at a mean temperature of 26.5±2.8 °C. 142 (28%) patients required emergent HARCH replacement for repair of acute type A aortic dissection. The overall mortality rate for patients undergoing HARCH was 6%. The incidence of PND and TND was 3.2% and 4.0% respectively. The incidence of dialysis dependent renal failure was 3.4%. There were no cases of paraplegia in the HARCH population.
In a multivariate logistic regression analysis, temperature was not found to be an independent risk during HARCH for mortality, PND, TND, or renal failure (Table 1).
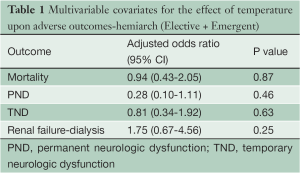
Full table
Elective vs. Emergent hemiarch
Preoperative demographics of the Elective and Emergent HARCH populations are displayed in Table 2. The Emergent cohort was younger (Emergent 55±13 years vs. Elective 60±14 years, P<0.01) and had a higher incidence of preoperative renal failure (18% vs. 4.8%, P<0.01) than the Elective group. Both groups had normal ventricular function and a low incidence of prior stroke.
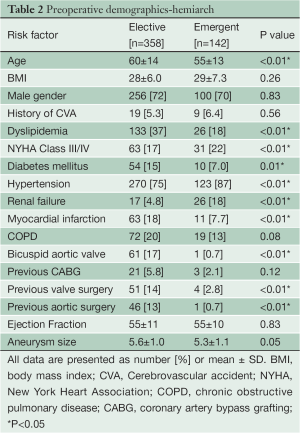
Full table
In the Elective group, concomitant aortic root replacement was performed in 186 (52%) patients, of which 55 (15%) were David V valve sparing procedures. In the Emergent group, concomitant aortic root replacement was performed in 47 (33%) patients, including 27 (19%) David V valve sparing procedures. 71 (20%) patients in the Elective group had undergone prior cardiac surgery via median sternotomy. Table 3 lists the concomitant procedures in both Elective and Emergent cases.
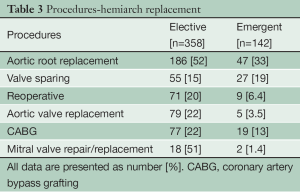
Full table
The temperature at the initiation of circulatory arrest was equivalent in the Elective and Emergent populations (Elective 26.6±2.8 °C vs. Emergent 26.3±2.8 °C, P=0.20). Operative mortality was 4.5% in the Elective group, and 11.1% in the Emergent group (P<0.01). The incidence of PND and TND in the Elective cases was 2.8% and 3.4% respectively. Emergent cases had a slightly increased incidence of PND (4.2%) and TND (5.6%), but this did not reach statistical significance. Post-operative renal failure was significantly higher in the Emergent population (Emergent 9.9% vs. Elective 0.8%, P<0.01). Cardiopulmonary bypass and myocardial ischemic times were no different between the groups. The duration of circulatory arrest was significantly shorter in the Elective cases (Elective 24±7 mins vs. Emergent 34±12 mins, P<0.01) (Table 4).

Full table
Total arch replacement
124 patients underwent TARCH under MHCA using uSACP at a temperature of 25.8±2.7 °C. 18 (15%) patients required emergent TARCH for repair of acute type A aortic dissection. The overall mortality for patients undergoing TARCH was 9.7%. The incidence of PND and TND was 2.4% and 5.6% respectively. The incidence of dialysis dependent renal failure was 3.4%. There were no cases of paraplegia in the TARCH population. In a multivariate logistic regression analysis, temperature was not found to be an independent risk during TARCH for mortality, PND, TND or renal failure (Table 5).
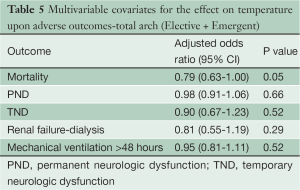
Full table
Elective vs. Emergent total arch replacement
Preoperative demographics of the Elective and Emergent TARCH populations are displayed in Table 6. The groups had similar preoperative risk factor profiles including age, hypertension, diabetes, myocardial infarction, stroke and renal failure. Both groups had normal ventricular function.
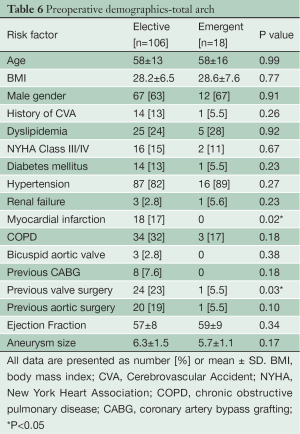
Full table
In the Elective group, concomitant aortic root replacement was performed in 39 (37%) patients, of which 12 (11%) were David V valve sparing procedures. In the Emergent group, concomitant aortic root replacement was performed in 4 (22%) patients, all of which were David V valve sparing procedures. 46 (41%) patients in the Elective group had undergone prior cardiac surgery via median sternotomy. Table 7 lists the concomitant procedures in both the Elective and Emergent cases.
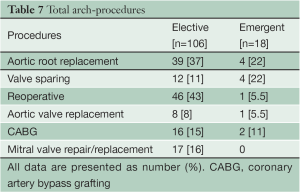
Full table
The temperature at the initiation of circulatory arrest was 26.6±2.7 °C in the Elective group and 27.2±2.4 °C in the Emergent group (P<0.01). Operative mortality was 10% in the Elective group, and 5.5% in the Emergent group (P=0.35). PND was lower in Elective cases (Elective 0.9% vs. Emergent 11%, P<0.01), but TND was equivalent (Elective 5.7% vs. Emergent 5.5%, P=0.79). The incidence of renal failure was low in both Elective (2.8%) and Emergent (5.5%) cases. Cardiopulmonary bypass and myocardial ischemic times were no different between the two groups. The duration of circulatory arrest was shorter in the Elective cases (Elective 53±16 mins vs. Emergent 61±19 mins, P=0.06) (Table 8).
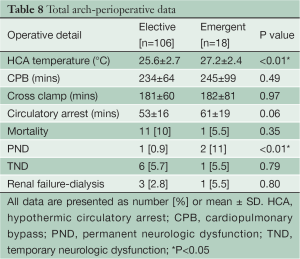
Full table
Discussion
The evolution of cerebral protection and circulation management strategies over the past four decades has enabled surgeons in the current era to successfully treat complex aortic arch pathology with excellent and reproducible outcomes. Historically, aortic arch surgery has been associated with neurologic injury and end-organ damage due to ischemic injury incurred during the period of circulatory arrest required to reconstruct the great vessels. Neurologic injury following arch surgery presents as either post-operative transient or permanent neurologic injury. PND is thought to be related to the site of arterial cannulation, while TND has been associated with inadequate cerebral protection (7,13,14).
The first successful series of arch replacements were performed with deep hypothermic circulatory arrest at 18 °C (1). Although not completely understood, profound hypothermia affords maximal cerebral and visceral organ protection during the period of circulatory arrest by reducing cellular metabolism. However, deep hypothermia is associated with adverse effects such as endothelial dysfunction, neuronal apoptosis, coagulopathy and renal failure (15-18). Furthermore, the safe duration of DHCA alone has been determined to be approximately 30 minutes, after which time ischemic cerebral injury occurs. This has translated into a 25% incidence of TND in patients who undergo arch replacement with DHCA alone (19-21).
DHCA served as the primary cerebral protection strategy for arch replacement until the late 1980’s, at which time surgeons began to utilize continuous cerebral perfusion in an attempt to reduce neurologic injury during arch reconstruction. The first large series using retrograde cerebral perfusion in conjunction with DHCA surfaced in the 1990’s. DHCA with retrograde cerebral perfusion (RCP) significantly reduced adverse neurologic outcomes and improved mortality in both elective and emergent arch replacements compared to DHCA alone (4-6). RCP is well recognized as a highly effective method of flushing embolic material from the cerebral circulation. However, experimental studies have demonstrated minimal cerebral blood flow during RCP, and an overall inability to support cerebral metabolism (22-24). This has translated into a TND incidence of 16% with the use of DHCA+RCP (6).
The first reports of DHCA with antegrade cerebral perfusion were also published in the early 1990’s (2,3). The technique of selective antegrade cerebral perfusion (most common nomenclature) is initiated during the period of circulatory arrest by introducing catheters into the innominate and left common carotid arteries, which provide continuous cerebral blood flow during arch reconstruction. SACP is the most physiologic method of cerebral protection, and changes the paradigm of total body circulatory arrest to lower body circulatory arrest as the brain, arms and spinal cord (via collateral circulation) are perfused. Multiple studies have demonstrated a reduction in the incidence of TND with DHCA+SACP compared to DHCA+RCP (7,14). Furthermore, antegrade cerebral perfusion has become the preferred method of cerebral protection in the current era by the majority of aortic surgeons, particularly for extended arch reconstructions requiring longer periods of circulatory arrest (25).
At Emory, our protocol for cerebral protection during arch replacement consists of right axillary artery cannulation, unilateral selective antegrade cerebral perfusion via the right common carotid artery (by clamping the innominate artery) and moderate hypothermic circulatory arrest (>22 °C). The results reported in this manuscript reflect our current outcomes with this technique. Patients undergoing elective HARCH at a mean temperature of 26.6 °C with relatively short circulatory arrest times had a mortality rate of 4.5%. Patients undergoing emergent HARCH replacement at a mean temperature of 26.3 °C for repair of acute type A aortic dissection had slightly longer circulatory arrest times and a mortality rate of 12%. Both groups had low rates of adverse neurologic outcomes. The incidence of post-operative renal failure was 9.9% in the Emergent type A group. We believe that this high rate of renal failure was related to preoperative dissection-induced renal malperfusion which was uncorrected by proximal aortic reconstruction alone, as opposed to inadequate end-organ protection from moderate hypothermia. The Elective group had a 0.8% incidence of renal failure.
We have also applied this technique to patients undergoing TARCH for more complex arch pathology. Our elective TARCH cohort was a complex population of patients with 43% having undergone prior cardiac surgery, and >50% requiring a concomitant procedure (root, valve, CABG, etc). Elective TARCH was performed at 25.6 °C with a mortality rate of 10%. Adverse neurologic outcomes (PND 0.9%, TND 5.7%) and renal failure rates (2.8%) were acceptable. Our experience with emergent TARCH for repair of acute type A aortic dissection is limited, consisting of 18 patients. These patients underwent TARCH at 27.2 °C with only a single mortality (5.5%). 2 patients suffered a postoperative stroke and a single patient each had TND and renal failure.
The data presented in this manuscript supports the use of MHCA and uSACP via the right axillary artery for aortic arch surgery. The use of moderate hypothermia did not adversely impact cerebral or visceral organ protection, as supported by the multivariate analyses conducted for both the HARCH and TARCH populations (Tables 1,4). This technique produces excellent neurologic outcomes for both HARCH requiring short circulatory arrest times (20-30 minutes), and TARCH, which requires prolonged periods of circulatory arrest (50-60 minutes). It avoids the placement of catheters into the ostia of the great vessels, which clutters the operative field and incurs both particulate and air embolism risk. As our experience with this technique has increased, we have conducted these operations under more moderate levels of hypothermia. Currently we perform emergent and elective HARCH and TARCH replacements at temperatures of 28-30 °C (9). The technique of MHCA and uSACP has enabled us to establish a successful, standardized method of circulation management in the treatment of patients with complex aortic arch pathology.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Bachet J, Guilmet D, Boudot B, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102:85-93; discussion 93-4. [PubMed]
- Kazui T, Inoue N, Yamada O, et al. Selective cerebral perfusion during operations for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53:109-14. [PubMed]
- Bavaria JE, Pochettino A, Brinster DR, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg 2001;234:336-42; discussion 342-3. [PubMed]
- Coselli JS, Lemaire SA. Experience with retrograde cerebral perfusion during proximal aortic surgery in 290 patients. J Card Surg 1997;12:322-5. [PubMed]
- Estrera AL, Miller CC, Lee TY, et al. Ascending and Transverse Aortic Arch Repair: The Impact of Retrograde Cerebral Perfusion. Circulation 2008;118:S160-6. [PubMed]
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9. [PubMed]
- Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90:547-54. [PubMed]
- Leshnower BG, Myung RJ, Thourani VH, et al. Hemiarch replacement at 28°C: an analysis of mild and moderate hypothermia in 500 patients. Ann Thorac Surg 2012;93:1910-5; discussion 1915-6.
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, et al. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-49. [PubMed]
- Khaladj N, Shrestha M, Peterss S, et al. Ascending aortic cannulation in acute aortic dissection type A: the Hannover experience. Eur J Cardiothorac Surg 2008;34:792-6; disussion 796.
- Ergin MA, Griepp EB, Lansman SL, et al. Hypothermic circulatory arrest and other methods of cerebral protection during operations on the thoracic aorta. J Card Surg 1994;9:525-37. [PubMed]
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1274-84. [PubMed]
- Hagl C, Ergin MA, Galla JD, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 2001;121:1107-21. [PubMed]
- Mora Mangano CT, Neville MJ, Hsu PH, et al. Aprotinin, blood loss and renal dysfunction in deep hypothermic circulatory arrest. Circulation 2001;104:I276-81. [PubMed]
- Cooper WA, Duarte IG, Thourani VH, et al. Hypothermic circulatory arrest causes multi-system vascular endothelial dysfunction and apoptosis. Ann Thorac Surg 2000;69:696- 702; discussion 703. [PubMed]
- Hagl C, Tatton NA, Khadalaj N, et al. Involvement of apoptosis in neurological injury after hypothermic circulatory arrest: a new target for therapeutic intervention? Ann Thorac Surg 2001;72:1457-64. [PubMed]
- Augoustides JG, Pochettino A, Ochroch EA, et al. Renal dysfunction after thoracic aortic surgery requiring deep hypothermic circulatory arrest: definition, incidence, and clinical predictors. J Cardiothorac Vasc Anesth 2006;20:673-7. [PubMed]
- Ergin MA, Galla JD. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994;107:788-97; discussion 797-9. [PubMed]
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9. [PubMed]
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurologic dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90; discussion 1891-4.
- Boeckxstans CJ, Fleming WJ. Retrograde cerebral perfusion does not protect the brain in non-human primates. Ann Thorac Surg 1995;60:319-27; discussion 327-8. [PubMed]
- Ye J, Yang L, Del Bigio MR, et al. Retrograde cerebral perfusion provides limited distribution of blood to the brain: a study in pigs. J Thorac Cardiovasc Surg 1997;114:660-5. [PubMed]
- Ehrlich MP, Hagl C, McCullough JN, et al. Retrograde cerebral perfusion provides negligibleflow through brain capillaries in the pig. J Thorac Cardiovasc Surg 2001;122:331-8. [PubMed]
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57. [PubMed]





