Step-by-step technique of robotic-assisted minimally invasive direct coronary artery bypass
Introduction
The benefit of grafting the left internal mammary artery (LIMA) to the left anterior descending artery (LAD) is thoroughly established (1). Several techniques, including minimally invasive and off-pump techniques, have emerged to accomplish LIMA to LAD (2-4). Cardiothoracic surgeons are advancing surgical techniques and driving technology to reduce patient suffering and improve patient quality of life (5-11). Recent studies over a 15-year period show that robotic-assisted minimally invasive direct coronary artery bypass (R-MIDCAB) and hybrid coronary revascularization (HCR) combined with percutaneous coronary intervention (PCI) provide good long-term outcomes in patients and provide an additional overall cost-benefit over traditional sternotomy coronary artery bypass grafting (CABG) secondary to decreased postoperative length of stay (12-16).
Our technique entails the robotic harvest of one or both internal thoracic arteries using the da Vinci® Surgical System (Intuitive Surgical® Inc., Sunnyvale, CA, USA) followed by direct LAD bypass through a minithoracotomy using the OCTOPUS® NUVO tissue stabilizer (Medtronic, Inc., Minneapolis, MN, USA). The ‘precision incision’ enables this procedure, which is mapped using three coordinates (5). The term is employed due to its reliable precision in identifying the optimal endoscopic port location, which allows for an unobstructed LIMA takedown and subsequent minithoracotomy site that provides clear and direct visualization for bypass grafting. This article describes in detail our standardized technique for robot-assisted coronary revascularization via minithoracotomy.
Methods
Critical step—building the team
The initial step is to assemble a dedicated team that will collaborate on all robotic cases (one team!). Achieving success in the operating room requires strong commitment, coordination, and communication from every member of the surgical team. It’s not just the surgeon who needs to master the use of the robot, but anesthesiologists and all surgical assistants must also become adept with new tasks, frequent instrument changes, the implications of CO2-induced alterations in the thoracic cavity, single right lung ventilation, and the crucial importance of anticipating or correcting the causes of robotic arm restrictions and movement limitations. Incremental learning is the key to success in all phases of this operation.
Step 1: patient positioning
Maintaining normothermia is a top priority and should be achieved before anesthesia induction and maintained throughout the entire operation. Additionally, external defibrillator pads should be applied to the patient, with one placed anteriorly over the right pectoralis major and the other posteriorly beneath the heart. The patient is positioned on the operative table with the left side aligned along the table’s edge, allowing the left shoulder to slightly overhang. This arrangement ensures unrestricted movement of the upper thoracic robotic arm. Occasionally, the upper robotic arm may encounter difficulty reaching the inferior part of the chest cavity due to the shoulder limiting its range of motion. However, advancing the trocar in the chest cavity or gently pressing on the left shoulder can enhance the arm’s maneuverability. Similarly, applying gentle pressure on the costal margin can improve the lower robotic arm’s movement as it extends superiorly into the chest. To safeguard the left arm, silicone padding is wrapped around the elbow, and a double-layered drawsheet is used as a sling to secure the limb alongside the patient. The right arm, also wrapped in silicone padding, is positioned securely on a slightly abducted arm board in the anatomical position. It has been observed that abducting the right arm, rather than adducting it, reduces postoperative numbness in the right upper extremity. This is because when the table tilts right-side down, the abdominal pannus tends to rest on the right arm. Each case is thoroughly prepared and draped to allow for seamless conversion to sternotomy, if necessary.
Step 2: the precision minithoracotomy incision
The precision minithoracotomy incision is located using a three-coordinate, standardized, step-by-step approach (5). The success of robotic-assisted LIMA to LAD bypass grafting via minithoracotomy depends on accurately placing the endoscopic port. The endoscopic port site for LIMA takedown will eventually serve as part of the thoracotomy site used for performing direct coronary anastomosis. To ensure a precise incision, mark the following landmarks on the applied Ioban 2 Antimicrobial Incise Steri-Drape (3M™, St. Paul, MN, USA) with a marking pen before making the incision: the sternal midline, the location of the LIMA, and the anterior edge of the diaphragm. The diaphragm is situated at the level where the lower edge of the sternum meets the xiphoid process. The final skin marking is critical and is determined by combining these three landmarks, resulting in an accurate incision (Figure 1). It is important to understand that this precise incision for the endoscopic port and subsequent thoracotomy site is typically based on these three coordinates rather than on a specific intercostal space or anatomic landmark, such as the midclavicular, or axillary lines. Although computed tomography scanning has been discussed in the literature as an alternative for mapping these three coordinates (5), we have found that it does not provide significant additional information compared to a standard antero-posterior and lateral chest X-ray (CXR).

Coordinate 1
For optimal camera placement and to ensure equal access and visibility to both the proximal and distal LIMA, align the endoscopic port along the chest’s longitudinal axis, (Figure 2), marking the midpoint between the suprasternal notch and the xiphoid process.
Coordinate 2
The endoscopic port should be aligned along the short (medial/lateral) axis. To orient the endoscopic port, begin by consulting the preoperative CXR to estimate the proportion of the hemithorax occupied by the cardiac silhouette. The distal portion of the LAD aligns to the heart’s apex, which in turn corresponds to the lateral edge of the cardiac silhouette on the CXR. Use this information to measure laterally from the mid-sternum and mark the skin. The cardiac silhouette can occupy anywhere from 40% to 100% of the left hemithorax. It is crucial to mark the Ioban chest drape precisely, using the left lateral bony thorax (rather than the lateral skin or fat) and the midline sternum as the boundaries of the hemithorax. Once the first and second coordinates are aligned, the mark is often located at the level of the nipple. As a rule, in a lean male, at the level of the nipple, the LAD target is approximately 70–80% down the distal LAD. In a heavier male, the breast mound is gently pulled toward the right shoulder with a 3M™ Steri-Drape. Conversely, the female breast is gently pulled laterally and flattened superiorly with Steri-Drape. The incision on the female breast is ultimately in its left lower inner quadrant.
Coordinate 3
Ensure that the anastomotic target on the LAD is clearly defined as either proximal, mid, or distal LAD. Use only coordinates 1 and 2 to position the endoscopic port over the mid-distal LAD. If the bypass target is more proximal or distal, it may require going an intercostal space above or below, respectively (Figure 3). A helpful tip is that for grafting more proximal LADs or the first diagonal/ramus intermedius, the incision should be positioned more medially. When applying the coronary stabilizer, it is important to note that the heart can be displaced inferiorly and medially about one inch in each direction but does not shift superiorly or laterally. Generally, the incision is below the nipple in male patients.
Step 3: endoscopic port placement
When placing the endoscopic port (Figure 2), it is important to remember that the heart could be just beneath the port placement site. To avoid undue injury, recheck lateral CXR or computed tomography (CT) scan views for the heart’s anterior position. It is crucial to take care and avoid injury to the heart.
Discontinue ventilation and positive end-expiratory pressure (PEEP), place a Veress needle superiorly at the midclavicular line; this allows CO2 insufflation to retract the heart posteriorly and medially from chest wall. Also, during port insertion, pay close attention to the electrocardiogram for changes as the tip of the obturator is passed through the chest wall. If the obturator is touching the heart, ensure that anesthesia has turned off the ventilator and intrathoracic CO2 is present. If electrocardiogram (ECG) changes [e.g., premature ventricular contractions (PVCs)] persist and heart is too close; consider another 8 mm port attempt more laterally. If there is concern that the heart is against the chest wall, it may be better to insert a finger first. Of note, it should be realized that one’s finger may increase the overall endoscopic port size which may cause subsequent increased loss of CO2.
The placement of the two additional ports for robotic surgery should be done under endoscopic guidance, ensuring they are aligned longitudinally in a straight line (Figure 4). These ports should be positioned 8–10 cm (approximately four fingerbreadths) above and below the endoscopic port, as the bony thorax allows. The bony thorax near the costal margin may sometimes necessitate positioning the lower port further posteriorly, which, though less ideal, seldom leads to significant instrument collisions with the camera (Figure 4). The lower port is used to elevate the bony thorax, thereby expanding the space between the pericardium and the anterior chest wall for the procedure. However, the upper port can only gently “burped”, as too much pressure during a lift will tear through intercostal muscles inferiorly along the line of the rib.
Anesthesia should always be alerted before attaching the CO2 as it causes physiologic changes similar to a “tension pneumothorax”. Usually 8–10 cm of CO2 pressure, but up to 14 cm can be used without difficulty. The space created using CO2 pressure is important for proper operative visualization, robotic arm mobility, and instrument changes. After placing the endoscope and instruments, another way to increase the anterior/posterior operative space is to release the medial and anterior pericardial fat attachments from the chest wall. This allows the CO2 to push the heart more posteriorly.
Step 4: opening the pericardium
The pericardium is opened before LIMA harvest to prevent inadvertent damage caused by pressure on the internal mammary artery (IMA) from robotic instruments, while the surgeon is focusing on opening the pericardium with the heart beating. There is a protective barrier of fat and muscle over the IMA in situ, versus harvesting first and having the IMA loosely suspended during pericardial opening which risks injury. When opening the pericardium, we use monopolar scissors and bipolar cautery. Using monopolar cautery too close to the heart may cause ventricular arrhythmias. In fact, ventricular tachycardia/fibrillation can occur when opening the pericardium and working close to the heart. Should an arrhythmia occur, the bedside team should remove all robotic instruments. For the most efficient defibrillation, anesthesia always keeps lidocaine hydrochloride (HCL) 100 mg out of the package and ready to inject. Additionally, it is best to reinflate the left lung. The circulator nurse simultaneously turns on the defibrillator to max joules and shocks as soon as possible (note, this should be part of routine drill, as max joules are not the rule in open cases).
By rotating the scope 90 degrees and directing it inferiorly, you can visualize the posterior pericardium. This area, located posterior to the phrenic nerve and free of fat, can be opened to facilitate the drainage of any blood that may accumulate during the anastomosis (Figure 5A). While this step is not essential, it helps manage potential bleeding from the chest wall, around the intracoronary shunt, or minor injuries to epicardial veins caused by the stabilizer. Next, the anterior pericardial fat is retracted laterally, and the anterior pericardium is opened just lateral to where the pulmonary artery is seen through the pericardium and over the left ventricle (Figure 5B). If any injury were to occur during opening the pericardium, injury to the left ventricle is better tolerated than injury to the right ventricle or injury to the pulmonary artery. Continue making the pericardial incision directly over the LAD to the diaphragm and proximally over the mid pulmonary artery to the aorta. Avoid making lateral incisions at the apex of the pericardium, as this can cause the heart to shift either laterally out of pericardium or upwards on its edge. Opening the pericardium widely serves three purposes:
- A broad pericardial incision ensures that the pericardial fat and the pericardial edge do not obstruct the view, especially when working through a small incision. This focus on the target minimizes the need for a larger thoracotomy to improve exposure.
- A wide pericardial opening enhances safety by reducing the risk of tamponade after the procedure.
- Finally, it ensures adequate drainage from the right side of the pericardium, addressing issues such as delayed inflammatory right sided atrial tamponade early in our experience.

When completed, the next step is to identify the target site of the LAD, hopefully beneath the endoscopic port. The optimal view of the target is achieved by aiming the endoscope slightly inferior and medial. This is because, upon removal of the robotic instruments with associated CO2 loss, the target will move superiorly and laterally, potentially by an inch or more in both directions. Next, place three small titanium Weck® Hemoclips (Teleflex Medical, Research Triangle Park, NC, USA) are placed in the epicardium using the small clip applier to mark the LAD target site. This will ensure that the target can be easily identified, and not confused with other vessels, when looking into the small thoracotomy. For reassurance, three hemoclips are placed, as it is difficult to obtain deep penetration into the epicardium without causing bleeding, and the superficial clip bites tend to fall off easily.
Step 5: LIMA harvest
It is important to constantly monitor the ‘pulsatility’ of the LIMA.
To start the robotic-assisted mammary takedown, employ the micro-bipolar forceps and permanent cautery spatula, with Intuitive Surgical® cautery at Classic 1 or Swift at 1–2. These settings help minimize the risk of damaging the LIMA. Higher wattages could burn a through the LIMA, whereas the specified settings are designed to cause only superficial, non-penetrating injuries. If bleeding is noted from avulsing collateral arteries or directly from the ITA, the use of a 7/0 Pronova suture (Ethicon, J&J, Raritan, NJ, USA) is the best next step to attempt containing the bleeding. Start the dissection where the LIMA is most easily seen, usually proximally. Next, determine the course of the LIMA by removing parietal pleura, layers of fat and muscle over the posterior LIMA. Seeing the entirety of the LIMA course leads to safer dissection. If significant fat is seen, remove the fat layers first. Then, dissect the endothoracic fascia on the posterior LIMA wall. The area on the posterior wall of the LIMA has few if any branch vessels.
After removal of multiple layers, any serpiginous coursing of the LIMA can be seen, further reducing the chance of LIMA injuries. Begin skeletonization distally, at the bifurcation, and proceed proximally. Circumferential dissection is best accomplished by incising the medial attachments first. Starting the LIMA dissection distally reduces the risk of bleeding from veins on either side of the LIMA. Our early experience showed that circumferential dissection proximal to distal led to increased venous bleeding. The principle of avoiding “dissecting into a hole” is a general surgical rule that also applies to robotic-assisted IMA takedown. The dissection should proceed with a gradual ascent anteriorly along the distance of the IMA rather than complete circumferential dissection at one area. When skeletonizing the IMA, it’s beneficial to keep some anterior attachments of the LIMA to the chest wall. This approach helps suspend the artery and protect it from accidental injury from a robotic arm. Manipulation of the IMA can lead to spasticity and increase the risk of clot formation, so preserving some of the larger distal branches can maintain better blood flow through the IMA and mitigate the procoagulant effects of manipulation. Avoid excessive traction or force, as the absence of haptic feedback can inadvertently lead to dissection of the LIMA wall. Ensuring that the LIMA remains a functional, pulsatile conduit is essential for successful CABG surgery.
Control any bleeding meticulously as blood can stain the tissue, making further dissection more difficult. Branches of the LIMA can be anywhere but expect them on both sides of each rib. Some surgeons primarily cauterize most branches and clipping only large branches. However, this surgeon prefers to clip the ‘branches’ proximally and cauterize distally (Figure 6). The dissection is complete proximally near the takeoff of the subclavian, but at a safe distance distal to the phrenic nerve which is intimately associated with the origin of the IMA.
Importantly, avoid risking injury to the phrenic nerve. If the phrenic nerve is obscured by fat, applying gentle downward pressure over the expected site of the phrenic nerve allows visualization of its course beneath the layer of fat superiorly. There are few bifurcating branches off the LIMA, however, frequently it is found approximately 2 cm short of the phrenic and IMA intersection. These bifurcating branches extend medially and posteriorly, which are difficult to see; thus, extra effort is needed to secure these branches.
Step 5a: bilateral IMA harvest
When harvesting both IMAs, start with the right IMA first. Position the upper port slightly more inferior and lateral than the usual to prevent injury to the LIMA. Use caution, as manipulating instruments upwards can exert substantial pressure on the LIMA while dissecting the proximal right IMA. During the initial dissection across the sternum towards the right IMA, ensure you maintain a dissection plane directly along the posterior sternal border.
Step 6: preparing for LIMA to LAD
The patient’s target heparinization should reach an activated clotting time of 300 seconds. A VG solution spray (2.5 mg verapamil, 1.25 mg nitroglycerin, 250 units heparin, 0.1 mL sodium bicarbonate, 150 mL ringer’s solution) is introduced via a #8 pediatric feeding tube through the lower port, and further dilates the LIMA. With the majority of LIMA dissection completed, but with IMA still minimally attached to the chest wall, detach a small distal segment of the LIMA, and tack the LIMA to the LAD target site using the ends of the 7-0 suture approximated with four Weck® hemoclips. A skeletonized LIMA will always rotate in a clockwise direction if not tacked to the epicardium. Although a pedicle graft is less likely to rotate, you will not know if this rotation occurs due to limited visualization through the minithoracotomy; hence, the tacking stitch is critically important. Another option is to clip the distal LIMA to the edge of the pericardium at the level of the LAD target location. Free the remaining IMA from the chest wall, clip and transect at the IMA bifurcation. At this time heparinization is warranted before transecting the LITA.
During port removal, carefully inspect all dissection areas and port sites for bleeding, as it is challenging to address any issues once the robotic ports are fully withdrawn. The final step with the robot involved inserting a clamped 19-F silastic flexible Blake drain (Ethicon) through the upper port, positioning it over the apex of the lung, and securing it in place (Figure 7). This drain will remove both air and fluid postoperatively. While the Blake drain can also be manually inserted at the end of the case, be mindful of the heart’s proximity.
Step 7: creating the precision incision
The incision can be any size, 4–12 cm, for surgeon comfort. The major benefit for patients is the less invasive robotic-assisted IMA harvest, not necessarily the size of the incision (Figure 7). TRS small ThruPort™ System soft tissue retractor (Edwards Lifesciences® Corp., Irvine, CA, USA), or the Alexis® Wound Protectors/Retractors (Applied Medical, Rancho Santa Margarta, CA, USA) is oriented within the converted endoscopic port/thoracotomy (Figure 8A). A width of at least two fingerbreadths is needed to introduce a TRS small ThruPort™ soft tissue retractor. Using a headlight and with a small appendectomy retractor, examine the markers/epicardial clips near the target site through the endoscopic port incision. Depending on the location of the target, adjust the endoscopic port/thoracotomy by extending it medially or laterally as needed. Keep in mind that it is always easier to move the heart medially.
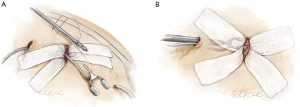
Make sure that the LIMA does not get caught in the soft tissue retractor by inspecting the chest before securing the adhesive ends. The OCTOPUS® NUVO tissue stabilizer has two components, which are visible separately in the field and subsequently connected within the minithoracotomy site. To stabilize the heart on the pericardium, place traction sutures on the lateral edge of the pericardium and pull them through the skin using a crochet hook via a 14-gauge angiocatheter. These sutures can help move the heart medially and keep the lung clear of the surgical area. The previously placed 7-0 tacking stitch to IMA prevents the IMA from twisting. Because there is significant slack in the LIMA conduit, another 7-0 suture should be used to retack the LIMA to the epicardium to effectively reduce the redundancy.
The positioning of the OCTOPUS® NUVO tissue stabilizer requires careful forethought (Figure 8).
- To ensure safe placement, insert your finger into the thoracotomy first and guide any instruments inserted through the lower port into your visual field. Use a long Kelly forceps clamp to gently pull the tubing of the tissue stabilizer retrograde through the precision incision and back out through the lower robotic port to attach to suction. Gently place the NUVO tissue stabilizer into the minithoracotomy (Figure 8A).
- Similarly, again, place the long Kelly clamp to make for easier insertion of the large OCTOPUS® NUVO pole into the chest cavity. Guide the pole from the lower port site to the minithoracotomy site with your fingertip to prevent damaging the heart. Given the heart’s proximity to chest wall, this precaution cannot be overemphasized. Assemble the pole and stabilizer within the chest while looking through the minithoracotomy site (Figure 8B). Once everything is in place, secure the coronary artery.
A helpful tip is to proactively fashion angled small Bulldog® clamps (Teleflex Medical, Research Triangle Park, NC, USA) with a string for ease of retrieval in the event of misplacement during anastomosis.
Step 8: anastomosis on the beating heart
Under direct visualization, position the suction stabilizer on the beating heart and secure the blunt-tip SaddleLoop 1541 (Quest Medical, Inc., Allen, TX, USA) around the proximal LAD, trimming away any surplus tape (Figure 9). Proceed with the dissect of the LAD target. It is preferred to use an under-sized coronary shunt should ischemia suddenly be an issue and so not damage the intima of the LAD during placement. Most anastomoses are performed using a 7-0 Prolene polypropylene suture (Ethicon, J&J). The suturing starts with three knots tied at the heel and ends midpoint on the medial side of the anastomosis. A nerve hook is used to tighten the suture before tying. Upon completion of the anastomosis, use of transit-time ultrasonic flow probe to perform an intraoperative functional assessment of flow measurements ensures graft patency. It would be ideal to have a hybrid operating room with cardiac catheterization capabilities as an alternative.
Step 9: closing
When the lung is inflated, position the pericardial fat over the LIMA anastomosis to shield it and prevent it from protruding into the minithoracotomy. Aim to ensure that the LIMA remains medial to lung as it inflates. The pericardium is left not reapproximated, as closing it could create excessive tension on the LIMA and significantly impede arterial flow. A 19-F Blake™ drain is inserted through the lower port site and positioned posteriorly over the diaphragm to facilitate effective drainage. Next, hold ventilation and suction the remaining blood from chest cavity. If the volume of blood is more than anticipated, re-examine the chest and reinsert the endoscope for visualization. If necessary, cover the enlarged thoracotomy with Vaseline™ (Covidien, Medtronic, Inc., Minneapolis, MN, USA) gauze to hold in the CO2.
Using a ligature carrier, place 2-0 Ethibond™ (Ethicon, J&J) pericostal sutures around the rib above and through the rib below to create a scaffold that prevents the lung from bulging out of the minithoracotomy. Next, approximate the pectoralis major muscle with 2-0 Vicryl polyglactic synthetic absorbable sutures (Ethicon, J&J) in a “figure of 8”, as necessary. Aesthetics are augmented with a 1-cm circumferential sliding cutaneous release just below the skin level and at the superficial fascial layer. This is done because the chest tissue laterally is pulled severely from having the left arm in a sling. In our experience, closures without this sliding release do not present a favorable appearance. The “sliding plasty” might have 1–2 interrupted 3-0 suture placed, to further approximate and close layers. Of note, generally women have no sutures placed above the pectoralis muscle regardless of the depth of breast tissue.
Step 10: pain management facilitating intraoperative extubation
The incisions mentioned above are injected with a mixture of 30 mL of lidocaine with epinephrine 0.5%—1:200,000, 30 mL of bupivacaine 0.25%, and 60 mL of ropivacaine with epinephrine 0.2%—1:600,000.
Intraoperative extubation can be beneficial for beating heart surgery (3) and minimally invasive robotic surgery.
Concluding remarks
This procedure is embraced by all who can perform it correctly; operative complications or graft failures will not be well tolerated because this procedure will be heavily scrutinized. Start with incremental learning; that is, move forward mastering various parts of the operation separately, and slowly advance with additional aspects of this precision incision procedure. For instance:
- If you are not a beating heart surgeon, perform LIMA to LAD anastomosis with the heart beating, while on pump.
- Take the opportunity to gain experience with the robotic set-up and perform LIMA harvest on your sternotomy cases. Of course, this requires discussion with the department chairperson & patient. Discuss the procedure with your patient beforehand, ensuring they provide written, informed consent. Explain that there are two methods to harvest the LIMA: robotically via three 8 mm port incisions or by using the Rultract® Skyhook Retractor (Rultract Incorporated, Cleveland, OH, USA) via a sternotomy. Most patients will prefer the robot!
- Initially, allocate an hour for setting up and harvesting the LIMA; gradually increase your robotic time before moving on with your operation via sternotomy. This approach allows you and your team to work at a comfortable pace, enhancing patient safety.
- Finally, start with a larger incision rather than a small minithoracotomy for your initial robotic experiences.
If you need to convert to a sternotomy, retuck the patient’s left arm at the side on the table rather than in a sling to avoid a curved chest incision. Conversion should be seen as a precaution for patient safety, not as a failure.
Robotic-assisted CABG is a promising technique that can achieve excellent results with a dedicated surgical team. This procedure offers significant benefits to patients when performed with commitment and skill.
Over the last 18 years (May 2005–June 30, 2024), almost half of all CABG interventions at Lankenau Medical Center, Wynnewood, PA were performed using the described technique for robotic-assisted beating heart surgery (n=2,852) (Figure 10) with a <2% sternotomy conversion rate. Overall results include, 88% extubated in the operating room, 17% transfusion rate, 17% incidence of postoperative atrial fibrillation, 0.44 % cerebral vascular accident, and a mortality rate of 1.2%. Our institution has experienced remarkable growth due to the collaborative efforts between cardiologists and cardiac surgeons. R-MIDCAB is not intended to compete with traditional sternotomy, but to provide a gold standard alternative to multivessel stenting, or offer an option to frail patients who otherwise would not tolerate sternotomy. HCR has increased volume for both cardiac surgery and interventional cardiology. Dedication to a multidisciplinary effort ensures that patients receive the best possible interventional option for myocardial revascularization in a totally unique and individualized patient-centered-model of care.

Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [Crossref] [PubMed]
- Subramanian VA, Loulmet DF, Patel NC. Minimally invasive coronary artery bypass grafting. Semin Thorac Cardiovasc Surg 2007;19:281-8. [Crossref] [PubMed]
- Gobran SR, Goldman S, Ferdinand F, et al. Outcomes after usage of a quality initiative program for off-pump coronary artery bypass surgery: a comparison with on-pump surgery. Ann Thorac Surg 2004;78:2015-21; discussion 2021. [Crossref] [PubMed]
- Trejos AL, Ross I, Scalesse C, et al. Preoperative evaluation of patient anatomy to increase success of robotics-assisted bypass surgery. Innovations (Phila) 2010;5:335-40. [Crossref] [PubMed]
- Sutter FP, Berry T, Wertan MC. Precision incision: robotic coronary revascularization via 3.9-cm minithoracotomy. Innovations (Phila) 2012;7:223-8. [Crossref] [PubMed]
- de Jong R, Jacob K, Jalali A, et al. Five-Year Outcomes After Hybrid Coronary Revascularization: A Single Center Experience. Innovations (Phila) 2021;16:456-62. [Crossref] [PubMed]
- Kiaii B, Teefy P. Hybrid Coronary Artery Revascularization: A Review and Current Evidence. Innovations (Phila) 2019;14:394-404. [Crossref] [PubMed]
- Amabile A, Komlo C, Van Praet KM, et al. Techniques for Robotic-Assisted Surgical Myocardial Revascularization. Surg Technol Int 2021;39:251-9. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Torregrossa G, et al. Multi-spectrum robotic cardiac surgery: Early outcomes. JTCVS Tech 2022;13:74-82. [Crossref] [PubMed]
- Yokoyama Y, Kuno T, Malik A, et al. Outcomes of robotic coronary artery bypass versus nonrobotic coronary artery bypass. J Card Surg 2021;36:3187-92. [Crossref] [PubMed]
- Jonsson A, Binongo J, Patel P, et al. Mastering the Learning Curve for Robotic-Assisted Coronary Artery Bypass Surgery. Ann Thorac Surg 2023;115:1118-25. [Crossref] [PubMed]
- Dokollari A, Sicouri S, Erten O, et al. Long-term clinical outcomes of robotic-assisted surgical coronary artery revascularisation. EuroIntervention 2024;20:45-55. [Crossref] [PubMed]
- Van den Eynde J, Vaesen Bentein H, Decaluwé T, et al. Safe implementation of robotic-assisted minimally invasive direct coronary artery bypass: application of learning curves and cumulative sum analysis. J Thorac Dis 2021;13:4260-70. [Crossref] [PubMed]
- Torregrossa G, Sá MP, Van den Eynde J, et al. Robotic-assisted versus conventional off-pump coronary surgery in women: A propensity-matched study. J Card Surg 2022;37:3525-35. [Crossref] [PubMed]
- Torregrossa G, Sá MP, Van den Eynde J, et al. Robotic hybrid coronary revascularization versus conventional off-pump coronary bypass surgery in women with two-vessel disease. J Card Surg 2022;37:501-11. [Crossref] [PubMed]
- Dokollari A, Sicouri S, Prendergrast G, et al. Robotic-Assisted Versus Traditional Full-Sternotomy Coronary Artery Bypass Grafting Procedures: A Propensity-Matched Analysis of Hospital Costs. Am J Cardiol 2024;213:12-9. [Crossref] [PubMed]












