Australian outcomes from heart transplantation in the machine perfusion era
Introduction
With an ever-increasing population of patients with end-stage heart failure (ESHF), global efforts are being made to address the disparity between the demand, which continues to rise, and the supply of donor hearts (1). This has manifested through the use of hepatitis C donors, older donors as well as marginal donors (2,3). As a by-product of this trend, machine perfusion strategies (either hypothermic, or normothermic) have emerged as promising additions to the armamentarium of the modern transplant unit.
Normothermic machine perfusion (NMP) allows for assessment of the donor heart following procurement and was originally used as a means to assess viability in marginal brain dead (BD) donor hearts (4). Previously, hearts from donation after circulatory death (DCD) donors were considered unsuitable for transplantation due to concerns surrounding the impact of warm ischemia during withdrawal of life support (WLS). However following a series of pre-clinical experiments, our unit performed the first heart transplant from a DCD donor using NMP in 2014 (5-8). The utilization of hearts from DCD donors represents arguably one of the most significant advancements in the efforts to expand the donor pool (9-12). The Transmedics Organ Care System Heart (OCS Heart) is currently the only commercially available NMP device. It perfuses the heart in a Langendorff fashion using donor blood as a part of its perfusate (8,9).
Static cold storage (SCS) of BD donor hearts continues to represent the most common modality of donor heart preservation. It is well known that increasing donor ischemic time (DIT), particularly beyond 4 to 6 hours, is a major risk factor for severe primary graft dysfunction (sPGD) and early post-transplant mortality (13-16). Large animal studies pioneered by Steen et al. (17) paved the way for hypothermic machine perfusion (HMP) to serve as a potential solution to prolonged donor ischemic times. The XVIVO Heart Assist Transport device (XHAT) (Gothenburg, Sweden) is the only commercially available HMP device; early results from clinical trials in Europe (18) and Australia/New Zealand (19) have shown promising outcomes. When mounted onto the XHAT, donor hearts are preserved at a temperature of approximately 8 ℃ and submerged in a perfusate consisting of a hyper-oncotic preservation solution as well as O negative blood; this perfusate is oxygenated and the coronaries are continuously perfused via an aortic cannula (17-19).
Australia is a country that is geographically dispersed—consequently, machine perfusion may play an important role in expanding the donor pool. In our unit NMP has been able to facilitate DCD heart transplantation since 2014. In 2021, HMP became available to our unit, initially as a part of an international trial (19). In this study we aim to report on heart transplantation outcomes in a unit where both forms of machine perfusion have been available. Our primary outcomes include overall survival and the incidence of sPGD.
Methods
Heart transplants that occurred in our institution (St Vincent’s Hospital Sydney) from January 2021 to February 2024 were included in this study. This time-period reflects the “machine perfusion era” when both HMP and NMP strategies have been available to our unit, in addition to ongoing use of SCS. Heart-lung block transplants were excluded from this study. Heart-kidney transplants were included.
Data was analyzed retrospectively; 161 hearts were transplanted during this time. For the purposes of comparison, three groups were identified: (I) DCD-NMP group (n=44)—heart transplants from DCD donors were included in this group, donor hearts were perfused using NMP (Transmedics OCS Heart); (II) BD-HMP group (n=38)—hearts transplanted from BD donors and preserved utilizing the XHAT HMP device were included in this group; (III) BD-SCS group (n=78)—this group reflected heart transplants from BD donors preserved utilizing traditional SCS. Following categorization, one heart transplant was excluded. This was a marginal BD heart that underwent assessment on the Transmedics OCS Heart NMP system (no incidence of sPGD and the patient remains alive after 2 years). Within the three groups a total of three patients were heart-kidney recipients, two in the DCD-NMP group and one in the BD-HMP group.
DCD donor inclusion criteria were as follows: Maastricht Category III donors, age ≤55 years, no previous cardiac history and normal trans-thoracic echocardiogram. Hearts retrieved from DCD donors were retrieved and assessed using the “Sydney Direct Procurement Protocol” which has been previously described—the last major change to this protocol occurred in January 2020 (9). Briefly, donor hearts were accepted for transplantation based on: (I) consideration of ischemic times; (II) adequate visual contractility of the right ventricle; and (III) observation of a down-trending and extracting lactate profile. Lactate extraction can be defined as serial point of care sampling demonstrating a venous lactate level (sampled from coronary effluent) being less than the arterial lactate level (sampled from coronary affluent) at a given time-point, with the overall lactate levels demonstrating a downward trend.
Standard and marginal criteria BD donors were considered. BD donor hearts were retrieved utilizing the XHAT HMP device if the anticipated donor ischemic time exceeded 6 hours (either due to donor location, transport time and/or recipient complexity); if this was not the case, BD donor hearts were retrieved utilizing SCS following administration of St Thomas’ cardioplegia (supplemented with glyceryl trinitrate and erythropoietin). The Australian experience and technique for mounting the XHAT HMP device, as well as monitoring and preparation has previously been described (19).
Recipients were selected based on blood-typing and cross match compatibility as well as predicted heart mass and clinical urgency. Recipients were consented to receive hearts utilizing machine perfusion strategies.
DCD timing definitions
We defined the total warm ischemic time (WIT) as the time from the WLS until the administration of cardioplegia to the donor heart during the organ procurement. In our unit the functional warm ischemic time (fWIT) is defined as the time from a systolic blood pressure (SBP) of <90 mmHg up to the administration of cardioplegia. Asystolic warm ischemic time (aWIT) is the time from donor cessation of circulation, until the delivery of cardioplegia (this includes a mandatory stand-down time, which is now at 5 minutes across Australia).
Donor ischemic/preservation time
In BD retrievals, the donor ischemic time is typically defined as the time from the application of aortic cross-clamp in the donor at the time of retrieval, until the time the cross-clamp is released in the recipient following implantation, “clamp on to clamp off”. The majority of this time is a reflection of SCS duration. When BD donor hearts are preserved in the XHAT HMP device, this same time period can be referred to as the donor preservation time (as the heart is not ischemic during HMP) (19). Therefore, for the purposes of comparison in this study, donor preservation time and donor ischemic time represent the same time-points.
Statistical analysis
Analysis was conducted using GraphPad Prism Version 10.2.0 (Graph Pad Software, San Diego, CA, USA). Significance was determined if the P value was <0.05. Normally distributed continuous data are expressed as a mean ± standard deviation with the Student’s t-test used to determine significance. Non-parametric data are expressed as a median (interquartile range) with Mann-Whitney analysis used to determine significance. In the comparison of multiple groups, one-way analysis of variance (ANOVA) was performed for normally distributed data, and a Kruskal-Willis test performed for non-parametric data with post-hoc testing performed to further explore any significance. Categorical variables were analyzed in contingency tables and significance was determined through Fisher’s exact test. Kaplan-Meier cumulative survival curves were generated to analyze survival with the log-rank test used to compare survival rates.
Results
Donor and recipient peri-operative characteristics
Table 1 details donor characteristics between the three groups. No significant differences in baseline characteristics including donor sex, age, weight and height were found. The donor preservation time in the BD-HMP group was significantly longer than the donor ischemic time in the BD-SCS group, and the OCS run time in the DCD-NMP group (361±89 vs. 208±47 and 249±49 min respectively, P<0.001). The OCS run time in the DCD-NMP group was also significantly longer than the donor ischemic time of the BD-SCS group. DCD-NMP and BD-HMP groups resulted in significantly more interstate retrievals compared to the BD-SCS group (61% and 74% vs. 19% respectively, P<0.001).
Table 1
| Characteristics | Donor characteristics by donor type/preservation strategy | |||
|---|---|---|---|---|
| DCD-NMP (n=44) | BD-HMP (n=38) | BD-SCS (n=78) | P value | |
| Sex (M:F) | 38:6 | 27:11 | 54:24 | 0.1 |
| Age (years), mean ± SD | 35±11 | 36±13 | 36±12 | 0.8 |
| Height (cm), mean ± SD | 174±16 | 176±10 | 176±10 | 0.6 |
| Weight (kg), mean ± SD | 83±19 | 82±16 | 82±14 | 0.9 |
| Mechanism of death, n [%] | – | |||
| Hypoxic brain injury | 18 [41] | 22 [58] | 43 [55] | |
| CVA | 13 [30] | 11 [29] | 25 [32] | |
| Traumatic brain injury | 12 [27] | 5 [13] | 9 [12] | |
| Brain tumor (US) | 0 [0] | 0 [0] | 1 [1] | |
| Volunteer assisted dying | 1 [2] | – | – | |
| Transmedics OCS run time/donor preservation time/donor ischaemic time (BD donor) (min), mean ± SD | 249±49‡ (Transmedics OCS run time) | 361±89†,‡ (donor preservation time) | 208±47 (donor ischaemic time) | <0.001 |
| Donor located outside of state, n [%] | 27 [61]‡ | 28 [74]‡ | 19 [24] | <0.001 |
†, significantly greater than DCD-NMP group on post-hoc analysis; ‡, significantly greater than BD-SCS group on post-hoc analysis. DCD-NMP, donation after circulatory death-normothermic machine perfusion; BD-HMP, brain dead-hypothermic machine perfusion; BD-SCS, brain dead-static cold storage; F, female; M, male; SD, standard deviation; CVA, cerebrovascular accident; BD, brain dead; OCS, organ care system.
Table 2 shows no significant difference in the baseline characteristics of sex, age, weight and height between the recipients of the three groups. Recipients in the BD-HMP group were significantly more likely to require a re-do sternotomy at the time of their transplantation compared to DCD-NMP and BD-SCS (66% compared to 39% and 36% respectively, P=0.0075), and had the highest proportion of recipients requiring explant of a durable ventricular assist device (VAD) (42%). There was a significant difference in cardiopulmonary bypass time and cross-clamp time between the groups; on post-hoc analysis, this difference was found to be in the BD-HMP group, which demonstrated significantly higher bypass and cross clamp times when compared to the DCD-NMP and BD-SCS groups. No significant differences were found when comparing the bypass and cross clamp times between the DCD-NMP and BD-SCS groups.
Table 2
| Characteristics | Recipients by donor type and preservation strategy | |||
|---|---|---|---|---|
| DCD-NMP (n=44) | BD-HMP (n=38) | BD-SCS (n=78) | P value | |
| Sex (M:F) | 37:7 | 29:9 | 60:18 | 0.2 |
| Age (years), mean ± SD | 52±11 | 51±15 | 51±14 | 0.09 |
| Height (cm), mean ± SD | 174±11 | 173±10 | 173±10 | 0.9 |
| Weight (kg), mean ± SD | 82±18 | 83±19 | 83±17 | >0.9 |
| Aeitiology of heart failure, n [%] | – | |||
| Dilated cardiomyopathy | 25 [57] | 18 [47] | 34 [44] | |
| Ischemic cardiomyopathy | 9 [20] | 9 [24] | 21 [27] | |
| Congenital | 2 [5] | 2 [5] | 5 [6] | |
| Viral myocarditis | 2 [5] | 1 [3] | 1 [1] | |
| Restrictive cardiomyopathy | 6 [14] | 0 [0] | 8 [10] | |
| Other | 0 [0] | 8 [21] | 9 [12] | |
| Mechanical support pre-transplant, n [%] | – | |||
| LVAD | 13 [30] | 16 [42] | 21 [27] | |
| VA ECMO | 2 [5] | 1 [3] | 0 [0] | |
| Impella | 2 [5] | 0 [0] | 0 [0] | |
| Redo sternotomy at time of transplant, n [%] | 17 [39] | 25 [66]†,‡ | 28 [36] | 0.0075 |
| Bypass time (min), median (IQR) | 145 (116–175) | 189 (139–233)†,‡ | 150 (119–183) | 0.0015 |
| Cross clamp time (min), median (IQR) | 82 (72–90) | 98 (83–111)†,‡ | 86 (74–100) | 0.0014 |
| Intensive care unit LOS (days), median (IQR) | 6 (3–10) | 5 (3–9) | 4 (3–9) | 0.7 |
| Hospital LOS (days), median (IQR) | 26 (13–42) | 34 (16–58) | 22 (13–39) | 0.2 |
| Permanent stroke, n [%] | 1 [2] | 1 [3] | 1 [1] | 0.8 |
| New renal failure requiring permanent dialysis, n [%] | 0 [0] | 3 [8] | 0 [0] | 0.01 |
| sPGD requiring mechanical support, n [%] | 3 [7] | 2 [5] | 4 [5] | 0.9 |
†, significantly greater than DCD-NMP group on post-hoc analysis; ‡, significantly greater than BD-SCS group on post-hoc analysis. DCD-NMP, donation after circulatory death-normothermic machine perfusion; BD-HMP, brain dead-hypothermic machine perfusion; BD-SCS, brain dead-static cold storage; M, male; F, female; SD, standard deviation; LVAD, left ventricular assist device; VA ECMO, veno-arterial extracorporeal membrane oxygenation; IQR, interquartile range; LOS, length of stay; sPGD, severe primary graft dysfunction.
Outcomes
Primary
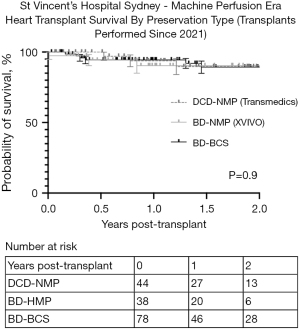
There were no significant differences in the rates of sPGD requiring extracorporeal membrane oxygenation (ECMO) between the DCD-NMP, BD-HMP and BD-SCS groups (7%, 5% and 5% respectively, P=0.9 not significant; Table 2). Furthermore, Figure 1 shows there to be no difference in overall survival between the three groups. For the groups DCD-NMP, BD-HMP and BD-SCS respectively: 30-day survival was 100%, 97% and 100%; 1-year survival was: 94%, 90% and 94%; 2-year survival was: 90%, 90% and 89% (P=0.9). There was no incidence of sPGD or mortality in the three heart-kidney recipients (all surviving >90 days).
Secondary
Table 2 demonstrates no significant differences in intensive care unit (ICU) or hospital length of stay (LOS) between the groups; the BD-HMP group had a significantly higher rate of permanent dialysis requirement post-transplant compared to the DCD-NMP and BD-SCS groups (13% vs. 0%). There were no differences in rates of permanent stroke between the groups (DCD-NMP: 2%, BD-HMP: 3%, BD-SCS: 1%, P=0.8 not significant). At time of hospital discharge following their transplant admission all three heart-kidney recipients had normal renal function and were free from dialysis.
DCD retrieval characteristics
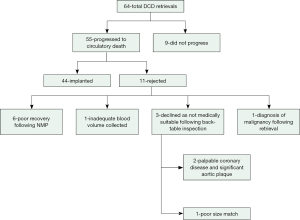
Figure 2 illustrates the total number of DCD retrievals since 2021. The DCD progression rate during this time was 86% (55/64) and the overall utilization of donor hearts for transplantation following progression was 80% (44/55). Poor recovery of the heart during NMP, and back-table inspection noting medical unsuitability following procurement, represented the most common reasons for rejection (82%, 9/11).
Retrieval timings for DCD donors as well as the OCS run time are noted in Table 3. Overall, no significant differences were found in retrieval parameters when comparing recipients of DCD heart transplants receiving ECMO for sPGD compared to those who did not, however there was a trend toward higher warm ischemic and OCS run times in DCD-NMP recipients who required ECMO for sPGD.
Table 3
| Retrieval characteristics | DCD retrieval parameters | |||
|---|---|---|---|---|
| Total (n=44) | Recipients without sPGD (n=41) | Recipients with sPGD (n=3) | P value | |
| Total warm ischaemic time (min), median (IQR) | 29 (23–32) | 29 (24–31) | 32 (23–38) | 0.4 |
| Functional warm ischaemic time (min), mean ± SD | 21±7 | 21±7 | 28±8 | 0.2 |
| Asystolic warm ischaemic time (min), median (IQR) | 13 (11–15) | 13 (11–15) | 15 (10–18) | 0.4 |
| Back-table cold ischaemic time (min), mean ± SD | 42±11 | 42±11 | 40±11 | 0.8 |
| Organ care system run time (min), mean ± SD | 249±49 | 246±49 | 279±57 | 0.4 |
DCD, donation after circulatory death; sPGD, severe primary graft dysfunction; IQR, interquartile range; SD, standard deviation.
Discussion
The addition of machine perfusion to the armamentarium of our transplant unit has had a positive impact in expanding the donor pool. Figure 3 demonstrates how machine perfusion strategies now account for 51% of all heart transplantation activity at our unit (82/160 heart transplants have occurred utilizing machine perfusion). DCD heart transplantation has previously been reported by our unit to account for 20% of our heart transplantation activity since 2014 (9). This contribution has grown, along with our experience, since 2021. This present study shows that DCD heart transplantation is responsible for 28% of our heart transplant activity.

DCD heart transplant experience
Schroder et al. (20) published the first randomized control trial involving DCD hearts and NMP. This study reported a 15% rate of post-transplant sPGD for recipients of DCD donor hearts compared to 5% in recipients of BD donor hearts. Our outcomes demonstrate a low rate of sPGD (7%) in DCD heart transplants since 2021, with no significant difference when compared to heart transplantation from HMP or SCS preservation strategies. There appears to be a learning curve associated with DCD heart transplantation, particularly when hearts are retrieved following a direct procurement pathway and then perfused on NMP. In describing their outcomes after 5 years of DCD heart transplantation at a single center, one of the early pioneers of DCD heart transplantation, the Papworth group (United Kingdom), reported an 18% ECMO rate for PGD for DCD hearts retrieved from using a direct procurement protocol (DPP) and NMP (10). However, in a later pilot study where the number of DCD heart transplant centers was expanded, inexperience in certain centers was thought to have played a role in an increased ECMO rate during this study period (40%) (21). Similarly, in our initial experience with DCD heart transplantation, the ECMO requirement for sPGD was 35% (5). As experience with DCD retrievals and NMP grew, more contemporary DCD heart transplant cohorts trended to have significantly lower rates of sPGD (8%) (9). With a 7% rate of sPGD in DCD heart transplants since 2021, this continues to be the case.
One of the key elements in the ameliorating sPGD rates in our experience has been the attention paid to minimizing aWIT; technical refinements as well as collaboration with donor hospital teams have helped in this regard. An aWIT >15 min has been consistently identified as a significant predictor of mechanical circulatory support (MCS) requirement for sPGD (5,9). In this study, it is once again noted that the median aWIT for donor hearts of recipients with sPGD is higher than those without sPGD (15 min compared to 13 min, though this is not significant likely due to the small number of patients who developed sPGD).
This need to heed attention to specific warm ischemic timings is also echoed by the Papworth group who believe outcomes are improved if an fWIT of <30 min is attained (the Papworth group define the start of fWIT when the donor SBP falls below 50 mmHg following WLS) (10,21). Given our findings when an aWIT is >15 min this recommendation is unsurprising and it is likely that below a SBP of 50 mmHg, diastolic perfusion of the coronaries is significantly impaired. Given unit dependent differences in the definition of fWIT, as well as lack of arterial line monitoring in certain situations, we suggest the aWIT as a potential universal parameter to be measured in all DCD retrievals.
Our experience with DCD heart transplantation and NMP continues to demonstrate equivalent survival and rates of sPGD when compared to BD preservation strategies.
Experience with HMP
As Table 1 highlights, machine perfusion has allowed our unit to successfully broaden its retrieval range with 61% of DCD retrievals being interstate. In the BD-HMP cohort, 74% of retrievals were either interstate, or international (1 retrieval from New Zealand).
Optimizing long distance procurement strategies is important to expanding the donor pool and is vital in a country like Australia. A highlight from our experience, which has been previously reported, was an instance where the XHAT HMP device was utilized to retrieve a donor heart approximately 1,400 km away from the recipient site with a total donor preservation time of 527 min and no incidence of post-operative sPGD (22). In France, Lebreton and Leprince describe a donor preservation time of 12 hours and 6 min utilizing the XHAT and procuring a donor heart across the Atlantic ocean with an excellent post operative outcome as the patient was transferred to ICU on minimal ionotropic support (23).
The mean donor preservation time was noted to be significantly longer in the BD-HMP group compared to the mean DIT in the BD-SCS group (361 vs. 208 min, P<0.001). Not only is this indicative of our experience in utilizing HMP for long distance procurement, it is also a by-product of implanting teams being able to select increasingly complex recipients whose operative complexity may have previously prolonged the DIT beyond 4-to-6 hours. This can be reflected as the HMP-BD group was significantly more likely to have recipients requiring a re-do sternotomy at the time of transplant as well as significantly longer cardiopulmonary bypass and cross-clamp times when compared to the other two groups (see Table 2).
Despite the increased recipient complexity and mean preservation time exceeding 6 hours in the BD-HMP group, there was no significant difference between all groups in: survival, rates of sPGD, LOS or incidence of permanent stroke. It was noted however that there was a significantly increased rate of post-transplant requirement for permanent dialysis in the BD-HMP group compared to the other groups [8% (3/38) compared to 0%, P=0.01]. However, for 2/3 cases, recipient pre-operative status likely played a role with two patients having pre-existing chronic renal disease (one of these patients also being a complex congenital re-do sternotomy).
The machine perfusion era and the future
These results demonstrate that in the machine perfusion era, results in our primary outcomes of survival and sPGD are equivalent across preservation modalities.
Our experience with DCD heart transplantation continues to demonstrate excellent results and with growing international experience, it should begin to be considered routine practice in large transplant centers. Whilst this paper discusses our experience with a DPP, normothermic regional perfusion (NRP) represents an alternative pathway for procuring DCD hearts and has also shown to confer survival and outcomes similar to standard BD donation (12). Ethical debate continues to surround NRP and it is currently unknown if there is a benefit to heart transplantation outcomes in comparing NRP to DPP strategies (24,25). This, along with determining the ideal preservation modality following NRP (NMP vs. HMP vs. SCS) represents areas for future research. Currently, as is also seen in our experience, NMP has an advantage in allowing for the long distant procurement of DCD hearts.
HMP has made a promising entry into the field of heart transplantation. With our results and international experience showing real-world data of successful heart transplantation with long distant procurement, the notion of international transplant programs may be a closer to becoming a reality. The question remains as to whether DCD heart transplantation, particularly from a DPP pathway could benefit from HMP. A large animal porcine study by Moeslund et al. (26) demonstrates a potential role for HMP in DCD heart transplantation, however this appears to be limited by warm ischemia (with two donor hearts experiencing an fWIT of >25 min found to demonstrate ischemic contractures and were not able to be weaned off bypass). Currently with no means of assessing DCD hearts for viability during HMP, this is a major limiting factor. Although limiting the acceptable fWIT time may represent a potential pathway for early clinical application, had this occurred in our clinical experience, NMP would have allowed for an assessment of the donor heart and could have potentially allowed for transplantation.
An International Society of Heart and Lung Transplantation consensus statement suggests that the ideal preservation temperature for donor hearts retrieved utilizing a hypothermic preservation strategy, is between 5–10 ℃ (27). The XHAT HMP device allows for preservation at 8 ℃ and also allows for ongoing oxygenated perfusion of the coronary arteries; however, there are also devices available that offer targeted temperature preservation without the element of machine perfusion, the SherpaPak Cardiac Transport System (S-CTS) (Paragonix Technologies, MA, USA) is an example of this and allows for preservation at approximately 5 ℃.
A retrospective study from Stanford (USA) compared a matched cohort of 62 and 124 recipients of donor hearts preserved with S-CTS or SCS respectively (28). In this study, the matched cohorts demonstrated a significantly longer mean donor organ ischemic time in the S-CTS group compared to the SCS group (246 vs. 227 min respectively, P=0.01) with no significant differences in survival or post-operative graft function (28). There appears to be a significant benefit in utilizing S-CTS for marginal donors—a retrospective multi-center subgroup analysis of outcomes from marginal donors by Moayedifar et al. (29) demonstrated the S-CTS group to have a significantly lower rate of sPGD compared to the SCS group (6.2% vs. 13.9% respectively, P=0.022) despite having a significantly higher number of donor hearts with a preservation time over four hours.
Whilst the mean donor organ ischemic time in the matched cohort reported by Zhu et al. (28) exceeds 4 hours, our reported experience with HMP involves a mean donor preservation time that well exceeds 6 hours (Table 1) with case reports of donor preservation times in excess of 8 hours, and 12 hours internationally (22,23). Whether this can be achieved using hypothermic strategies without perfusion remains to be seen. In considering alternative hypothermic strategies, the question arises: how much does hypothermic perfusion play a role in the results of our study, and, could this potentially have been achieved with static temperature control devices such as S-CTS instead?
Conclusions
Machine perfusion strategies are an important addition to the armamentarium of the modern transplant unit. It has allowed for the expansion of the donor pool for both the types of donors that can be used (NMP) as well as in increasing the range of retrievals (HMP and NMP). For BD donors, HMP allows for donor hearts to be preserved significantly longer than SCS with no differences in survival or sPGD. Overall, there are no differences in survival when comparing DCD-NMP, BD-HMP and BD-SCS groups. Rates of sPGD are low and are not significantly different between the groups (7%, 5% and 5%, respectively).
Acknowledgments
We would like to acknowledge and thank—Prof. David McGiffin & Prof. David Kaye from The Alfred Hospital (Melbourne, VIC, Australia) who were primary investigators of the NIHP trial.
Funding: None.
Footnote
Conflicts of Interest: Y.J. is supported by a National Heart Foundation PhD scholarship. P.M.: modules in kind from XVIVO and Transmedics (unrelated to submitted work); Novartis and Amgen (grant paid to institution), Novartis, Boehringer-Ingelheim and Astra-Zeneca (Advisory board membership). The other authors have no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akintoye E, Alvarez P, Shin D, et al. Changing Demographics, Temporal Trends in Waitlist, and Posttransplant Outcomes After Heart Transplantation in the United States: Analysis of the UNOS Database 1991-2019. Circ Heart Fail 2021;14:e008764. [Crossref] [PubMed]
- Dharmavaram N, Hess T, Jaeger H, et al. National Trends in Heart Donor Usage Rates: Are We Efficiently Transplanting More Hearts? J Am Heart Assoc 2021;10:e019655. [Crossref] [PubMed]
- Huckaby LV, Hickey G, Sultan I, et al. Trends in the utilization of marginal donors for orthotopic heart transplantation. J Card Surg 2021;36:1270-6. [Crossref] [PubMed]
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577-84. [Crossref] [PubMed]
- Chew HC, Iyer A, Connellan M, et al. Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J Am Coll Cardiol 2019;73:1447-59. [Crossref] [PubMed]
- Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015;385:2585-91. [Crossref] [PubMed]
- Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant 2015;15:371-80. [Crossref] [PubMed]
- Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant 2014;14:1744-52. [Crossref] [PubMed]
- Joshi Y, Scheuer S, Chew H, et al. Heart Transplantation From DCD Donors in Australia: Lessons Learned From the First 74 Cases. Transplantation 2023;107:361-71. [Crossref] [PubMed]
- Messer S, Cernic S, Page A, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2020;39:1463-75. [Crossref] [PubMed]
- Siddiqi HK, Trahanas J, Xu M, et al. Outcomes of Heart Transplant Donation After Circulatory Death. J Am Coll Cardiol 2023;82:1512-20. [Crossref] [PubMed]
- Louca J, Öchsner M, Shah A, et al. The international experience of in-situ recovery of the DCD heart: a multicentre retrospective observational study. EClinicalMedicine 2023;58:101887. [Crossref] [PubMed]
- Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014;33:327-40. [Crossref] [PubMed]
- Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg 2007;133:554-9. [Crossref] [PubMed]
- Taylor DO, Edwards LB, Boucek MM, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report--2004. J Heart Lung Transplant 2004;23:796-803. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Steen S, Paskevicius A, Liao Q, et al. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J 2016;50:193-200. [Crossref] [PubMed]
- Nilsson J, Jernryd V, Qin G, et al. A nonrandomized open-label phase 2 trial of nonischemic heart preservation for human heart transplantation. Nat Commun 2020;11:2976. [Crossref] [PubMed]
- McGiffin DC, Kure CE, Macdonald PS, et al. Hypothermic oxygenated perfusion (HOPE) safely and effectively extends acceptable donor heart preservation times: Results of the Australian and New Zealand trial. J Heart Lung Transplant 2024;43:485-95. [Crossref] [PubMed]
- Schroder JN, Patel CB, DeVore AD, et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N Engl J Med 2023;388:2121-31. [Crossref] [PubMed]
- Messer S, Rushton S, Simmonds L, et al. A national pilot of donation after circulatory death (DCD) heart transplantation within the United Kingdom. J Heart Lung Transplant 2023;42:1120-30. [Crossref] [PubMed]
- Emmanuel S, Muthiah K, Tardo D, et al. Advances in cardiac machine perfusion: Exceeding 8 hours from procurement to implant without requiring extracorporeal membrane oxygenation. J Heart Lung Transplant 2023;42:1766-7. [Crossref] [PubMed]
- Lebreton G, Leprince P. Successful heart transplant after 12 h preservation aboard a commercial flight. Lancet 2024;403:1019. [Crossref] [PubMed]
- Entwistle JW, Drake DH, Fenton KN, et al. Normothermic regional perfusion: Ethical issues in thoracic organ donation. J Thorac Cardiovasc Surg 2022;164:147-54. [Crossref] [PubMed]
- Wall AE, Fiedler A, Karp S, et al. Applying the ethical framework for donation after circulatory death to thoracic normothermic regional perfusion procedures. Am J Transplant 2022;22:1311-5. [Crossref] [PubMed]
- Moeslund N, Ertugrul IA, Hu MA, et al. Ex-situ oxygenated hypothermic machine perfusion in donation after circulatory death heart transplantation following either direct procurement or in-situ normothermic regional perfusion. J Heart Lung Transplant 2023;42:730-40. [Crossref] [PubMed]
- Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: A consensus statement. J Heart Lung Transplant 2020;39:501-17. [Crossref] [PubMed]
- Zhu Y, Shudo Y, He H, et al. Outcomes of Heart Transplantation Using a Temperature-controlled Hypothermic Storage System. Transplantation 2023;107:1151-7. [Crossref] [PubMed]
- Moayedifar R, Shudo Y, Kawabori M, et al. Recipient Outcomes With Extended Criteria Donors Using Advanced Heart Preservation: An Analysis of the GUARDIAN-Heart Registry. J Heart Lung Transplant 2024;43:673-80. [Crossref] [PubMed]






