Electrical graft assessment of machine-perfused hearts donated after circulatory death
Introduction
The introduction of normothermic ex situ heart perfusion (ESHP) has led to successful enlargement of the cardiac donor pool using marginal and donated after circulatory death (DCD) hearts (1). However, DCD hearts experience significant injury as a result of functional warm ischemic time (FWIT). During normothermic ESHP, donor hearts are transported in a beating state, allowing for viability assessment which is essential to prevent graft failure after transplantation (2). Current assessment strategies rely on visual contractility evaluation of an unloaded heart and lactate trends in the perfusate. However, lactate profiles are not sensitive enough to assess graft functionality (3,4) and visual inspections are inherently subjective.
Furthermore, hemodynamic parameters, such as ejection fraction, cannot be assessed on the only currently available clinical device for ESHP [Transmedics Organ Care System (OCS) Heart] due to perfusion in Langendorff mode (2,5). Therefore, more functional tools with direct feedback have to be developed to assess cardiac performance during ESHP (6).
We previously reported on the use of electrical mapping as novel assessment strategy for hearts on normothermic ESHP in a porcine DCD model (7). In this experimental setup, we showed that electrophysiological parameters, such as potential voltage, could distinguish between hearts with different warm ischemic profiles whereas lactate profiles could not. Therefore, we argued that electrical markers could serve as a novel adjunct strategy to assess cardiac function. In the current study, the use of this electrical mapping approach is described in the clinical setting of cardiac transplantation with human DCD hearts transported on ESHP.
Methods
Patients were allocated as DCD donors for cardiac transplantation and provided consent for research in accordance with the Dutch law on organ donation. Organ recipients provided informed consent for additional measurements on the donor hearts prior to transplantation (MEC 2023-0035) and for anonymous use of their medical details for scientific publication on DCD transplantation (MEC 2020-0106), as approved by the institutional medical ethics committee.
Direct DCD heart procurement and ex situ perfusion
Donor hearts were procured in standard manner after declaration of death and a five-minute no-touch period following circulatory arrest. FWIT started when systolic blood pressure dropped below 50 mmHg and ended with cardioplegic flush of the donor heart (Figure 1). Hearts were revived on the OCS as previously described (2,5), and transported to the Erasmus Medical Center. Blood gas and lactate levels were measured in the blood-priming perfusate and corrections were made accordingly (5).

Electrical mapping
Upon arrival at our hospital, high-resolution epicardial mapping of the donor heart was performed on the OCS, before cooling of the donor heart and cardioplegia administration (Figure 1) (7,8). Sterile drapes were placed on the OCS and a steel wire was carefully wrapped around the aorta as the indifferent electrode. The pacemaker’s stimulation function was temporarily turned off to record the heart’s intrinsic electrophysiological characteristics. The mapping equipment consisted of a 192-electrode array (electrode diameter 0.45 mm, inter-electrode distance 2 mm) (Delft University of Technology, the Netherlands) connected to a custom-made computerized mapping system (Figure 2). The epicardial surface of the heart was systematically mapped by moving the electrode-array over the left (LV) and right ventricle (RV) (Figure 3). The electrode was positioned parallel to the posterior interventricular coronary artery, starting at the atrioventricular groove. The electrode was shifted from the posterior to the anterior wall of each ventricle. Five seconds were recorded at every mapping position.
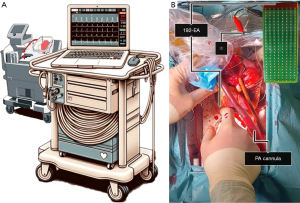

Detailed methods on electrical data analysis are provided in the Supplementary file (Appendix 1). In short, color-coded voltage maps were created by quantifying peak-to-peak amplitudes of unipolar extracellular potentials at each electrode, and local activation time maps were created to study abnormalities in myocardial conduction. Medians and interquartile ranges of potential voltage, slope, and conduction velocity and the amount of low-voltage potentials (voltage: LV <2.0 mV and RV <1.0 mV) and conduction block (inter-electrode time difference ≥12 ms) were calculated for the LV and RV of each donor heart.
Results
Patient and allograft characteristics
Epicardial mapping was performed on ten ex situ perfused DCD hearts [median donor age 38 years (31–44 years), 40% male] with a median FWIT of 15 minutes (15–18 minutes) and time on ESHP of 274 minutes (228–334 minutes). All transplanted hearts showed a decreasing lactate trend during ESHP (Table 1) and good visual contractile function. One additional DCD heart (donor age: 28 years) that was rejected for transplantation due to an increasing lactate trend (1.2 mmol/L/h starting from 5.6 mmol/L/h) was used to allow for comparison with the transplanted hearts.
Table 1
| Ex situ perfusion time | Lactate |
|---|---|
| Reperfusion | 6.4±1.6 mmol/L |
| 1 hour | 6.4±1.5 mmol/L |
| 2 hours | 5.8±1.5 mmol/L |
| 3 hours | 5.0±1.4 mmol/L |
| Gradient | −0.6±0.5 mmol/L/h |
Values are presented as average ± standard deviation. DCD, donated after circulatory death.
Electrical function
Table 2 provides electrophysiological characteristics of ten DCD hearts on ESHP. In total, more than one hundred thousand unipolar potentials were recorded from the ventricular surface. No procedure-related complications occurred during the mapping and sterility was maintained in all cases. Average recording time was 213±41 seconds.
Table 2
| Characteristics | Transplanted DCD hearts (n=10) |
|---|---|
| Unipolar potential characteristics | |
| Voltage (mV) | |
| LV | 15.7 (14.0–17.4) |
| RV | 11.3 (8.3–11.9) |
| Low-voltage (%) | |
| LV | 0.2 (0.1–0.3) |
| RV | 0.2 (0.1–1.1) |
| Slope (−V/s) | |
| LV | 1.7 (1.4–2.2) |
| RV | 1.0 (0.7–1.1) |
| Conduction characteristics | |
| Conduction velocity (cm/s) | |
| LV | 106 (98–113) |
| RV | 89 (77–92) |
| Conduction block (%) | |
| LV | 0.5 (0.0–1.5) |
| RV | 2.9 (1.8–5.2) |
Values are presented as median (interquartile range). DCD, donated after circulatory death; LV, left ventricle; RV, right ventricle.
Median voltage was 15.7 mV (14.0–17.4 mV) and 11.3 mV (8.3–11.9 mV) for the LV and RV respectively, and the amount of low-voltage potentials was minimal (Table 2). Figure 4 provides exemplary voltage maps, demonstrating how potential voltages are displayed during ESHP. Dark red colored areas indicate sites from which low-voltage potentials were recorded.

Conduction velocity was higher in the LV [106 cm/s (98–113 cm/s)] compared to the RV [89 cm/s (77–92 cm/s)] and in both ventricles only minimal areas of conduction block were found [LV: 0.5% (0.0–1.5%), RV: 2.9% (1.8–5.2%)] (Table 2).
Electrical function of the rejected donor heart
Unipolar potential voltages of the DCD heart that was rejected for transplantation due to increasing lactate levels (LV: 19.4 mV, RV: 10.4 mV) were comparable to the ten transplanted DCD hearts. Moreover, this heart presented without areas of low-voltage. Also, conduction velocity was similar (LV: 107 cm/s, RV: 99 cm/s) and the amount of conduction block (LV: 0%, RV: 0.4%) was not higher in this heart.
Post-operative outcomes
After ESHP and electrical mapping, donor hearts were cooled on OCS and transplanted, after which patients were transferred to the intensive care unit (ICU). Median recipient age was 26 years (25–51 years), the majority were male (70%), and five (50%) patients had a left ventricular assist device in situ prior to transplantation. All patients were alive thirty days after transplantation, with a median ICU stay of 7 days (4–10 days). Two patients required extracorporeal membrane oxygenation (ECMO) support four and five days, respectively, post-transplantation due to acute cardiac stunning and poor biventricular function. One of these hearts showed electrical abnormalities (reduced potential voltages, slopes and conduction velocities, and more conduction block) during electrical mapping on ESHP, which was not seen in the other heart (Table S1).
Discussion
Given the suboptimal graft assessment experience with lactate, there is an urgent need for additional functional parameters for hearts on ESHP (3,4). This is the first clinical report of an electrical mapping procedure of DCD hearts on normothermic ESHP, which has great potential as a novel adjunct for graft quality assessment.
Clinical experience
Epicardial mapping was performed on ten hearts on ESHP without adverse events, demonstrating safety of the technique, and supported by our group’s experience in over 1,500 patients undergoing cardiac surgery (8). Mapping can be performed in three-to-four minutes, providing real-time visualization of electrograms and color-coded potential voltage maps. Differences in potential voltage distribution across the epicardium could reveal localized areas of ischemic damage, whereas lactate levels only indicate global metabolic (dys)function.
The rationale to use electrophysiological determination of ischemia is based on its use in daily practice in diagnosing myocardial infarction. Nevertheless, such an approach has not yet been used in the setting of organ preservation. In general, a high amount of low-voltage potentials or conduction block is indicative of myocardial ischemic injury (9) which has been corroborated by our previous work on ischemic porcine hearts on ESHP (7). Within the current study, no high amounts of low-voltage potentials or areas of conduction block were observed, as expected in healthy human donor hearts. Of interest, one heart got rejected for transplantation on the basis of an increasing lactate trend, yet, electrical function was comparable to the transplanted hearts. This raises the question of whether this heart could have successfully been transplanted, as others have already showed successful transplantation of hearts with rising lactates (3). In such cases, we believe that functional parameters, like electrophysiological properties, can aid in decision-making regarding the organ’s suitability for transplantation. We hypothesize that an elevation in lactate levels concomitant with impaired electrical function, indicated by the prevalent occurrence of low-voltage potentials, should alert the surgical team to consider rejection of the heart. Otherwise, hearts could possibly be accepted for transplantation despite increasing lactate levels. Thus, addition of a real-time mapping technique could aid in graft evaluation, particularly for marginal donors and hearts with increasing lactate trends, and possibly allow more transplantations.
Future research is required to investigate whether electrical markers could predict the need for ECMO support after cardiac transplantation. Yet, this is also contingent on the recipient’s condition.
Feasibility
The equipment needed for the presented technique should be available in any center with a clinical electrophysiology lab. Mapping was performed in the recipient hospital as it was difficult to bring the current equipment on organ transplantation transport. In the future, we hope to develop a portable mapping device, where electrodes can be temporarily fixated covering the entire epicardial surface of the donor heart, so electrical assessment is continuously available and hands-free.
The presented electrophysiological characteristics can be interpreted as normal electrical function of DCD hearts on ESHP. However, more experience in a larger series is needed to validate the specificity of our technique, also including transplanted hearts with an increasing lactate trend during ESHP.
Conclusions
Our high-resolution electrical mapping approach of DCD hearts on ESHP is safe and feasible, and may serve as novel additional diagnostic tool for assessing graft function of marginal donor hearts.
Acknowledgments
The authors would like to kindly thank L.E. Sluijter-Rozendaal, B. Wouterse, A. Akman, J. Sjatskig, P.C. van de Woestijne and M.S. van Schie for their contribution. The authors used OpenAI’s DALL·E software to create parts of figures. After using this software, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Langmuur SJJ, Amesz JH, Veen KM, et al. Normothermic Ex Situ Heart Perfusion With the Organ Care System for Cardiac Transplantation: A Meta-analysis. Transplantation 2022;106:1745-53. [Crossref] [PubMed]
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577-84. [Crossref] [PubMed]
- Cernic S, Page A, Messer S, et al. Lactate during ex-situ heart perfusion does not predict the requirement for mechanical circulatory support following donation after circulatory death (DCD) heart transplants. J Heart Lung Transplant 2022;41:1294-302. [Crossref] [PubMed]
- Bona M, Wyss RK, Arnold M, et al. Cardiac Graft Assessment in the Era of Machine Perfusion: Current and Future Biomarkers. J Am Heart Assoc 2021;10:e018966. [Crossref] [PubMed]
- Tsukashita M, Naka Y. Organ care system for heart procurement and strategies to reduce primary graft failure after heart transplant. Operative Techniques in Thoracic and Cardiovascular Surgery 2015;20:322-34.
- Neyrinck A, Amarelli C, Dalvindt M, et al. editors. Consensus Conference Highlights - Machine perfusion in cardiothoracic transplantation. ESOT TLJ 30; 2022; Prague: The European Society for Organ Transplantation.
- Amesz JH, Bierhuizen MF, Langmuur SJ, et al. Ex-Situ Electrical Mapping of Machine Perfused Hearts Donated after Circulatory Death. The Journal of Heart and Lung Transplantation 2023;42:S377.
- Yaksh A, van der Does LJ, Kik C, et al. A novel intra-operative, high-resolution atrial mapping approach. J Interv Card Electrophysiol 2015;44:221-5. [Crossref] [PubMed]
- Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ Res 1997;80:124-38. [Crossref] [PubMed]






