Bilateral internal thoracic artery grafting in robotic beating-heart totally endoscopic coronary artery bypass: 10-year outcomes
Introduction
The use of bilateral internal thoracic artery (BITA) grafts in coronary artery bypass grafting (CABG) surgery has been associated with better outcomes than single ITA grafting in patients with multivessel coronary artery disease (CAD). Robotic off-pump totally endoscopic coronary artery bypass (TECAB) represents the least invasive form of surgical coronary revascularization. In specialized centers it can offer swift recovery and good long-term outcomes and graft patency. The sternal-sparing robotic-assisted approach allows for harvesting of both ITA grafts with no risk of sternal wound complications. In this study, we present the 10-year follow-up on our series of patients undergoing robotic TECAB with bilateral ITA grafts at a single institution in the setting of a multi-spectrum robotic cardiac surgery practice. In addition to demonstrating the safety and feasibility of this approach, we present good mid-term clinical outcomes as well as early graft patency in patients undergoing hybrid revascularization.
Methods
Study population
Eight hundred and seventy-one patients underwent robotic-assisted, beating-heart TECAB between 7/2013 and 4/2024 (single surgeon/institution). Of these, 406 underwent BITA grafting and are the subject of this review. Clinical outcomes were retrospectively reviewed from our prospectively collected database with Institutional Review Board approval (#18-0742; date of approval 4/28/2020). Angiographic data were reviewed in patients undergoing percutaneous coronary intervention (PCI) after TECAB in the setting of hybrid coronary revascularization (HCR). Mid-term clinical data were collected from annual patient contact (phone calls, email, or family/cardiologist contact). Major adverse cardiac and cerebrovascular events (MACCEs) included cardiac-related mortality, myocardial infarction (MI), repeat cardiac surgery, repeat revascularization to the surgical culprit vessel, and/or stroke.
Selection and surgical technique
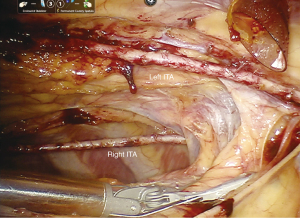
We have previously described our robotic-assisted, beating-heart TECAB surgical technique in detail (1-3). See Figure 1 for example of BITA after skeletonized harvesting. We detailed our technique for multi-vessel grafting with BITA, specifically, in two recent publications (4,5). Patients are considered for TECAB on an all-comer basis, according to the recommendations of the heart-team as previously reported. Patients are referred (either by cardiologist or self-referral) typically seeking a sternal-sparing option for coronary revascularization. All are discussed within the heart team, specifically with interventional cardiology in cases of multi-vessel disease to ensure hybrid revascularization can be achieved when needed. The only absolute exclusion criteria for TECAB are a fused left chest, or emergency surgery.
Statistical analysis
Continuous variables were tested for normality using the Shapiro-Wilk test. Those with normal distribution are expressed as mean ± standard deviation, and those without as median (interquartile range). Categorical and sequential variables are expressed as the number and percentage of patients. Kaplan-Meier analysis was applied for mid-term survival rate and freedom from major adverse cardiac events (MACEs). A P value <0.05 was considered statistically significant. The statistical analyses were conducted using IBM SPSS 25 (IBM, Inc., Chicago, IL, USA).
Results
Of a total of 871 patients undergoing TECAB during the study period, 470 patients (54%) had multi-vessel grafting and of these, 406 (86%) received BITA grafts. Demographics are shown in Table 1. The mean Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) score was 1.47%±2.2%. The mean age of the patient cohort was 67±9 years, with 16% female. Comorbidities in the patient cohort included those 87% with hypertension, 26% with prior MI, and 22% with chronic kidney disease. Thirty-nine percent had diabetes and 38% of these (15% overall) had insulin-dependent diabetes mellitus (IDDM). The mean body mass index (BMI) was 29±5 kg/m2. Thirty-nine percent of patients had a BMI ≥30 kg/m2, 11% had a BMI ≥35 kg/m2, and 4% had BMI ≥40 kg/m2. Three patients had undergone prior heart surgery [one of these prior CABG with vein grafts to obtuse marginal (OM) and right coronary artery (RCA)].
Table 1
| Variables | Data (n=406) |
|---|---|
| Age (years) | 67±9 |
| Female gender | 66 [16] |
| STS score (%) | 1.47±2.2 |
| BMI >30 kg/m2 | 158 [39] |
| Hypertension | 354 [87] |
| Diabetes mellitus | 158 [39] |
| IDDM | 60 [15] |
| Chronic renal failure | 88 [22] |
| Renal failure on dialysis | 10 [2.5] |
| COPD | 20 [4.9] |
| EF ≤40% | 79 [20] |
| Atrial fibrillation | 39 [9.6] |
| Prior cerebrovascular accident | 37 [9.1] |
| Prior MI | 104 [26] |
| Prior PCI | 147 [36] |
| Previous cardiac surgery | 3† [0.7] |
| Angina | 202 [50] |
| Left main disease ≥70% | 73 [18] |
Data are presented as mean ± SD or n [%]. †, one previous coronary artery bypass. STS, Society of Thoracic Surgeons; BMI, body mass index; IDDM, insulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Three hundred and forty-two patients (84%) underwent TECAB grafting (two grafts), 15% had three grafts, and two patients had four grafts. The mean number of grafts per patient was 2.2±0.4 (see Table 2 regarding graft details). The most common grafting configuration (63%) was LITA-LAD, and right ITA (RITA) to an anterior non-LAD or lateral wall target. The LAD was grafted with LITA in 65% and RITA in 35%. In the first 5 years, the distal anastomosis was performed using the C-Port Flex A anastomotic device for a majority of the grafts (45% overall), and in the latter 5 years, a robotic suture technique was used in the majority of patients due to the device being taken off the market. A total of seven patients required the use of cardiopulmonary bypass (CPB) via peripheral femoral cannulation, with five for hemodynamic support due to difficult target exposure and two for planned concomitant intra-cardiac procedures. The remaining cases were all completed off-pump, on a beating-heart. The intraoperative blood transfusion requirement was 6%. The mean operative time was 313 min. There were no conversions to sternotomy. See Table 3.
Table 2
| Variables | Data (n=878) |
|---|---|
| Grafts per patient | 2.2±0.4 |
| LITA flow (cc/min) | 84±42 |
| LITA PI | 1.4±0.4 |
| RITA flow (cc/min) | 74±35 |
| RITA PI | 1.6±0.5 |
| Anastomosis technique | |
| Anastomotic device (C-Port Flex A) | 397 [45] |
| Sutured (7-0 Pronova) | 466 [53] |
| U-clips | 15 [2] |
| Redo anastomosis | 6 [0.7] |
Data are presented as mean ± SD or n [%]. LITA, left internal thoracic artery; PI, pulsatility index; RITA, right internal thoracic artery; SD, standard deviation.
Table 3
| Variables | Data (n=406) |
|---|---|
| Operative time (min) | 313±58 |
| TECAB graft | |
| Two grafts | 342 [84] |
| Three grafts | 62 [15] |
| Four grafts | 2 [0.5] |
| CPB use | 7 [1.7] |
| Inotrope requirement | 5 [1.2] |
| Intraoperative BTF use | 24 [5.9] |
| Conversion | 0 |
| OR extubation | 154 [38] |
Data are presented as mean ± SD or n [%]. TECAB, totally endoscopic coronary artery bypass surgery; CPB, cardiopulmonary bypass; BTF, blood transfusion; OR, operating room; SD, standard deviation.
The mean hospital and intensive care unit (ICU) length of stay (LOS) were 2.44±0.83 and 1.22±0.62 days, respectively. Eight percent of patients required postoperative blood transfusion. Thirteen percent had new-onset atrial fibrillation, 1.2% had pericarditis, and 3.4% had postoperative acute kidney injury (AKI). There were no wound infections. There was one incidence of MI and one stroke, respectively (0.2% each). Three patients (0.7%) required return to theatre for bleeding which necessitated sternotomy in two cases. Mortality occurred in five patients with an observed/expected (O/E) ratio of 0.89 (O/E: postoperative mortality incidence/preoperative STS PROM). See Tables 4,5. At 30-day follow-up, the readmission rate was 5%, and 3% of patients had a pleural effusion requiring thoracentesis. The mean time to return to full activities and work was 14 and 17 days, respectively. All patients were reached for mid-term clinical follow-up (mean follow-up 51±36 months). The longest follow-up was 10.4 years. All-cause mortality was 13%, and cardiac-related mortality was 2.5%. See Figures 2,3. Repeat cardiac surgery occurred in six patients. Two patients underwent redo-CABG, one occurred 3.5 years after TECAB (despite having a patent LITA-LAD at the time of hybrid RCA stenting), and one 3.5 years after TECAB due to high-grade left main disease. One patient required aortic valve replacement (AVR) 2 years postoperatively. Three patients with pre-operative ischemic cardiomyopathy went on to advanced surgical therapy [two patients had a heart transplant, and one required left ventricular assist device (LVAD) placement]. All had patent grafts. The incidence of MI and unplanned PCI was 1.7% and 5.9%, respectively. Of the 18 patients undergoing repeat percutaneous intervention, only one was for a failed surgical graft. Freedom from angina was 96%, and freedom from MACE was 92% (Table 6). See Figures 2-4 for Kaplan-Meier survival curves demonstrating key mid-term outcomes.
Table 4
| Postoperative variables | Data (n=406) |
|---|---|
| Extubation within 6 hours | 325 [80] |
| Post-operative BTF use | 33 [8.1] |
| Chest tube drainage total (mL) | 651±296 |
| Chest tube drainage 24 hours (mL) | 588±244 |
| ICU LOS (days) | 1.22±0.62 |
| Hospital LOS (days) | 2.44±0.83 |
| Mortality | 5 [1.2] |
| Mortality, O/E | 0.89 |
| Readmission | 21 [5.2] |
| Mean time to return to full activities (days) | 14±8.6 |
| Mean time to return to work (days) | 17±13 |
Data are presented as n [%], mean ± SD, or ratio. BTF, blood transfusion; CU, intensive care unit; LOS, length of stay; O/E, observed/expected; SD, standard deviation.
Table 5
| Postoperative variables | Data (n=406) |
|---|---|
| Reintubation | 6 [1.5] |
| Prolonged ventilation (>24 hours) | 5 [1.2] |
| Wound infection | 0 |
| AKI | 14 [3.4] |
| Pericarditis | 5 [1.2] |
| Pleural effusion | 12 [3.0] |
| Clinical MI | 1 [0.2] |
| New atrial fibrillation | 54 [13] |
| Sepsis | 1 [0.2] |
| Stroke or TIA | 1 [0.2] |
| Re-exploration for bleeding | 3 [0.7] |
Data are presented as n [%]. AKI, acute kidney injury; MI, myocardial infarction; TIA, transient ischemic attack.
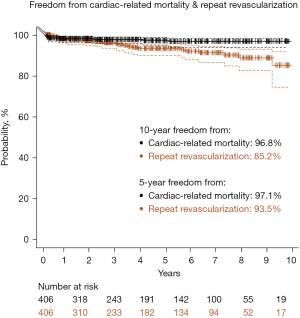
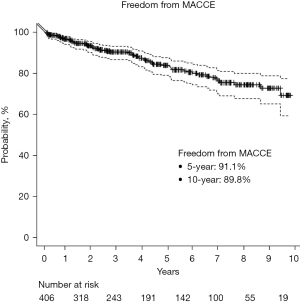
Table 6
| Mid-term variables | Data (n=406) |
|---|---|
| Average time to follow-up (months) | 51±36 |
| All-cause mortality | 54 [13] |
| Cardiac-related mortality | 10 [2.5] |
| Repeat cardiac surgery | 5 [1.2] |
| MI | 7 [1.7] |
| Repeat angiography | 56 [14] |
| Unplanned PCI | 24 [5.9] |
| Unplanned PCI in culprit (surgical) vessel | 11 [2.7] |
| Unplanned PCI in culprit (PCI) vessel | 4 [1.0] |
| PCI for failed graft | 1 [0.2] |
| Freedom from angina | 391 [96] |
| Freedom from MACCEs | 375 [92] |
Data are presented as mean ± SD or n [%]. MI, myocardial infarction; PCI, percutaneous coronary intervention; MACCE, major adverse cardiac and cerebrovascular event; SD, standard deviation.
Two hundred and two patients (50%) were selected for an advanced hybrid coronary revascularization (AHCR) strategy (BITA grafting plus PCI). Of these, 94% had surgery first, followed by staged PCI. The mean time to catheterization for these patients undergoing PCI after TECAB was 2.3±5.3 months. Overall, graft patency was 98% (271/278 grafts). Overall, RITA patency was 96% (130/136 grafts). Overall, LITA patency was 99% (141/142 grafts), with 100% LITA-LAD patency (Table 7).
Table 7
| Early graft patency | Data (n=135) |
|---|---|
| Time to catheterization (months) | 2.3±5.3 |
| Total IMA grafts imaged | 278 |
| Graft patency (n=278) | 271 [98] |
| LITA patency (n=142) | 141 [99] |
| RITA patency (n=136) | 130 [96] |
| LITA-LAD patency (n=85) | 85 [100] |
Data are presented as mean ± SD, n, or n [%]. PCI, percutaneous coronary intervention; TECAB, totally endoscopic coronary artery bypass; IMA, internal mammary artery; LITA, left internal thoracic artery; RITA, right internal thoracic artery; LAD, left anterior descending; SD, standard deviation.
Discussion
We present a series of 406 patients undergoing robotic-assisted beating-heart TECAB with BITA grafting over a 10-year period, with good early and mid-term outcomes. This is part of a multi-arterial grafting (MAG) strategy, where we employ an exclusive ITA conduit strategy in all patients undergoing multi-vessel TECAB. To our knowledge, this is the largest reported series of BITA grafting via a totally-endoscopic, off-pump robotic approach. The notable benefits of this sternal-sparing technique in coronary revascularization as it relates to BITA use are: maintaining an all-arterial grafting strategy, achieving complete revascularization (using a hybrid revascularization strategy in half the patients), and eliminating the risk of sternal wound infection (SWI).
MAG and total arterial grafting (TAG) in surgical revascularization has been shown to have superior outcomes compared to single arterial grafting (SAG) (6). Multiple studies have demonstrated improved long-term survival and lower rates of repeat revascularization/other MACE with MAG compared to SAG (7-11). Rocha et al. in a multi-center, propensity-matched study comparing CABG patients who received TAG (~5% overall) to those who did not demonstrate better survival and lower MACCEs/MI at 8 years in the TAG group (12). In another recent meta-analysis of 44 studies comparing MAG to SAG, median survival (at 17.5 and 11.6 years, respectively), both overall and event-free, were higher in the MAG group (13).
Specifically with regard to BITA grafting, several studies have demonstrated long-term survival benefits compared to single vessel ITA (SITA). This includes in overall CABG populations (14-17), elderly patients (18,19), low ejection fraction (EF) (20), reported “high-risk” patients (21), and importantly, those with diabetes (9,22,23). Despite this well-documented evidence, the use of both TAG (5% overall in the aforementioned Rocha et al. study) and BITA remain remarkably low across the US (6,8,21). When considering conduit choice for a second arterial graft in addition to the LITA, several studies have demonstrated a long-term survival benefit with use of RITA as a 2nd arterial conduit, even when compared to the radial artery (RA) (13,24,25).
As is known, the only randomized trial of BITA vs. SITA in CABG (ART trial) failed to show superiority of BITA over SITA grafting in the ‘intent-to-treat’ analysis, however, this can be attributed to multiple factors including a significant crossover rate, as well as the inclusion of RA grafts in the SITA group. In the as-treated analysis, BITA grafting was indeed shown to be superior to SITA in this trial (26). Because of this, the ROMA trial is currently underway. It is a prospective, randomized multicenter study evaluating MAG using RITA or RA as a second arterial conduit. The results of this trial, as well as the equivalent study with female participants only (ROMA women), will provide more information on best practices in total and MAG (7).
Frequently cited reasons for hesitancy in using BITA grafting include concern for SWI and longer operative time required for BITA harvesting, in addition to those specifically related to the technical aspects of using the RITA such as: lack of familiarity, concern over the length of RITA, or grafting configuration (6,9,23). Sternal wound complications/infections remain the primary concern with BITA. Although several studies have shown no difference in SWI rates between SITA and BITA, including in diabetic patients (22,23,27), there have been studies with contradictory findings and therefore this debate is ongoing. Given these potential difficulties of RITA harvesting and use in traditional CABG surgery, utilization of this conduit received only a class IIB indication in both the European Society of Cardiology/European Association for Cardiothoracic Surgery (ESC/EACTS) as well as the STS current guidelines, whereas the use of a RA graft received a class I indication in targets with high-grade stenosis (28).
However, we believe that the potential drawbacks of routine BITA harvesting are “easily” mitigated using a robotic endoscopic surgical approach, or indeed any approach in which the sternum is left intact. Given this, the inclusion/exclusion criteria in this series were not limited by the concern for SWIs. In our practice, patients are considered on a nearly all-comer basis for TECAB, including those with obesity, diabetes (including insulin-dependent), women, elderly, etc. In this series, diabetics and patients with a BMI above 35 constituted 39% and 11% of patients, respectively. The link between complete revascularization and improved long-term outcomes is well-documented (29,30). Glineur et al. evaluated patients with >2-vessel CAD undergoing multivessel grafting with BITA plus either additional arterial Y grafts or vein grafts. In comparison, they found at 14 years the BITA + Y arterial grafting group had better survival compared to the BITA + vein group (31). Bakaeen et al. importantly found that in BITA grafting, placing a second ITA to another major left coronary target was associated with higher long-term survival, whereas use of vein grafts did not have this survival benefit (32). In our TECAB practice, we ensure complete revascularization in patients with multi-vessel disease through either a third (or rarely, fourth) arterial graft via a sequential or Y technique with BITA; and/or through an AHCR strategy. AHCR achieves sternal-sparing complete revascularization in patients with multi-vessel CAD through the integration of two ITA grafts with PCI (the latter typically to right-sided targets). We recently described a series of 156 patients undergoing AHCR with good early and mid-term clinical outcomes, and an overall early graft patency of 98%. In that study, the mean residual SYNTAX score after AHCR was 2.6±3.0. In another previous report looking at our AHCR TECAB experience, we found that 86% of patients had complete/near-complete revascularization (defined by a residual SYNTAX score <8), which was associated with improved survival and lower MACCEs (5). In the present study, 202 patients (50%) were assigned to an AHCR strategy.
Another cited reason for low use of BITA is lack of familiarity in harvesting RITA/BITA conduits. This potentially could be even more true in a minimally invasive/endoscopic setting if one is not comfortable with these techniques. We recommend mastering RITA/BITA harvesting in the open setting prior to transitioning to a robotic-assisted endoscopic approach. In addition, we believe that the use of the robot as frequently as possible for multiple different types of procedures can be important in the success of a robotic multi-vessel TECAB program (33). In our practice, this strategy has allowed the robot to become simply another instrument/tool that all theatre staff are very familiar and comfortable with. The learning curve for robotic BITA harvesting can differ based on the surgeon’s experience, however, once LITA harvesting is mastered it should not be difficult to cross the midline and harvest the RITA as the technique is near identical. The epicardial Endowrist StabilizerTM (Intuitive Surgical, Sunnyvale, CA, USA), which is an essential tool for robotic endoscopic grafting, is also useful for aiding in RITA harvesting by gently depressing anterior mediastinal structures when crossing the midline. While our ITA harvesting technique has not changed significantly over time, our anastomotic technique has. We transitioned from a coronary stapler device, which was available until about 2018, to a robotic-assisted sutured technique for all anastomoses once the stapler device was removed from the market. We use a 7-0 double-armed Pronova suture and routinely shunt all anastomoses (34). Again, the importance of the stabilizer in the overall performance of robotic beating-heart TECAB cannot be understated or over-emphasized.
In addition to robotic-assisted CABG, minimally invasive approaches are being increasingly used for multi-vessel grafting and include mini-thoracotomy BITA grafting with or without thoracoscopic assistance to increase visualization (35). BITA use in this realm has been reported with good outcomes, although its widespread use (like in traditional CABG) remains low (36-38). These approaches, similar to TECAB, offer the significant advantages of reduced recovery time while still being able to employ bilateral ITAs in the context of sternal-sparing complete revascularization (± AHCR). We believe, however, that the totally endoscopic off-pump approach described here with robotic assistance remains the least invasive form of surgical coronary revascularization. Some of the advantages of adding the robotic approach include the enhanced visualization and ergonomics for the surgeon as well as the potential for offering the procedure to patients with less than ideal anatomy (e.g., morbidly obese).
We prefer to skeletonize ITA conduits. In the open setting, skeletonization has been shown to have equivalent or even lower rates of SWIs, increase length of the conduit, and increase anastomotic flow rate (39-41). There have also been studies comparing patency between skeletonized vs. pedicled ITA grafts, with some studies finding no difference in patency between the two techniques, and others finding higher patency in pedicled grafts. However, the advantage of the superior visualization from the robotic approach cannot be understated in this regard, as it lends itself to high precision and careful maneuvering around the conduit, and thus facilitates skeletonization.
Our approach to graft configuration when using BITA in robotic multi-vessel TECAB is that we aim to maintain a LITA-LAD configuration as much as possible and most commonly use the in-situ RITA for a second important left coronary target. If the lateral wall cannot be reached with in-situ RITA, then we use LITA for this and place the RITA on the LAD. If the in-situ RITA will reach neither, it is configured as a Y graft off of the LITA to a lateral wall target. There have been multiple studies comparing between RITA-LAD vs. LITA-LAD patency, revealing no difference in mid/long-term patency or clinical outcomes (42-45). We looked at this in our robotic TECAB population in a recent study comparing between these two groups and found no difference in early clinical outcomes or graft patency between RITA vs. LITA-LAD. In a propensity-matched sub-analysis of only patients with multi-vessel disease undergoing BITA grafting in each group, we found no differences in patency or midterm clinical outcomes (46).
Finally, a word about the future. Robotics in cardiac surgery is growing overall as patients increasingly request sternal-sparing options and as surgeons are introduced to robotics early in their surgical training. Increased adoption and utilization of this technology by our specialty will not only allow us to respond to the desires of our patients, but also be competitive in this era of rapidly growing transcatheter options in the treatment of heart diseases. One potential and increasingly discussed direction forward is designating coronary revascularization as a sub-specialty within cardiac surgery. In this realm, TAG with more common use of BITA and use of the least invasive approaches and technologies, i.e., off-pump bypass and robotics, could be offered on a routine basis. It would also allow for the re-engagement of our partners in the surgical device industry so that technology, such as the epicardial stabilizer (necessary for TECAB or any off-pump robotic procedure) or automated anastomotic devices (something we believe facilitates TECAB), can be available again, and new technologies in coronary revascularization can once more be developed.
Conclusions
We conclude that in the setting of a dedicated robotic cardiac program, patients with multi-vessel CAD can undergo bilateral ITA grafting using the least invasive surgical approach: robotic beating-heart TECAB. In addition to excellent early clinical outcomes and swift recovery, we demonstrate good midterm outcomes at 10 years and good graft patency comparable to traditional CABG, with the added benefit of no sternal wound complications using this sternal-sparing approach.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: H.H.B. is a proctor for Intuitive Surgical. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic off-pump totally endoscopic coronary artery bypass in the current era: report of 544 patients. Eur J Cardiothorac Surg 2022;61:439-46. [Crossref] [PubMed]
- Balkhy HH, Nisivaco SM, Hashimoto M, et al. Robotic Total Endoscopic Coronary Bypass in 570 Patients: Impact of Anastomotic Technique in Two Eras. Ann Thorac Surg 2022;114:476-82. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic Multivessel Endoscopic Coronary Bypass: Impact of a Beating-Heart Approach With Connectors. Ann Thorac Surg 2019;108:67-73. [Crossref] [PubMed]
- Kitahara H, Hirai T, McCrorey M, et al. Hybrid coronary revascularization: Midterm outcomes of robotic multivessel bypass and percutaneous interventions. J Thorac Cardiovasc Surg 2019;157:1829-1836.e1. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic advanced hybrid coronary revascularization: Outcomes with two internal thoracic artery grafts and stents. JTCVS Tech 2022;16:76-88. [Crossref] [PubMed]
- Torregrossa G, Amabile A, Williams EE, et al. Multi-arterial and total-arterial coronary revascularization: Past, present, and future perspective. J Card Surg 2020;35:1072-81. [Crossref] [PubMed]
- Gaudino M, Alexander JH, Bakaeen FG, et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031-40. [Crossref] [PubMed]
- Rocha RV, Tam DY, Karkhanis R, et al. Multiple Arterial Grafting Is Associated With Better Outcomes for Coronary Artery Bypass Grafting Patients. Circulation 2018;138:2081-90. [Crossref] [PubMed]
- Ren J, Royse C, Srivastav N, et al. Long-Term Survival of Multiple Versus Single Arterial Coronary Bypass Grafting in Elderly Patients. J Clin Med 2023;12:2594. [Crossref] [PubMed]
- Alsaleh D, Sun E, Alzahrani A, et al. Multiple arterial versus single arterial grafting in patients with diabetes undergoing coronary artery bypass surgery. JTCVS Open 2023;13:119-35. [Crossref] [PubMed]
- Tam DY, Rocha RV, Fang J, et al. Multiple arterial coronary bypass grafting is associated with greater survival in women. Heart 2021;107:888-94. [Crossref] [PubMed]
- Rocha RV, Tam DY, Karkhanis R, et al. Long-term Outcomes Associated With Total Arterial Revascularization vs Non-Total Arterial Revascularization. JAMA Cardiol 2020;5:507-14. [Crossref] [PubMed]
- Magouliotis DE, Fergadi MP, Zotos PA, et al. Differences in long-term survival outcomes after coronary artery bypass grafting using single vs multiple arterial grafts: a meta-analysis with reconstructed time-to-event data and subgroup analyses. Gen Thorac Cardiovasc Surg 2023;71:77-89. [Crossref] [PubMed]
- Dorman MJ, Kurlansky PA, Traad EA, et al. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation 2012;126:2935-42. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Taggart DP, Benedetto U, Gerry S, et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N Engl J Med 2019;380:437-46. [Crossref] [PubMed]
- Barili F, Onorati F, D'Errigo P, et al. Bilateral Internal Thoracic Arteries Improve 10-Year Outcomes of Coronary Artery Bypass Grafting. Ann Thorac Surg 2023;116:52-60. [Crossref] [PubMed]
- Navia D, Espinoza J, Vrancic M, et al. Bilateral internal thoracic artery grafting in elderly patients: Any benefit in survival? J Thorac Cardiovasc Surg 2022;164:542-9. [Crossref] [PubMed]
- Itoh S, Kimura N, Adachi H, et al. Is Bilateral Internal Mammary Arterial Grafting Beneficial for Patients Aged 75 Years or Older? Circ J 2016;80:1756-63. [Crossref] [PubMed]
- Galbut DL, Kurlansky PA, Traad EA, et al. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: a propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg 2012;143:844-853.e4. [Crossref] [PubMed]
- Saran N, Locker C, Said SM, et al. Current trends in bilateral internal thoracic artery use for coronary revascularization: Extending benefit to high-risk patients. J Thorac Cardiovasc Surg 2018;155:2331-43. [Crossref] [PubMed]
- Stefil M, Dixon M, Benedetto U, et al. Coronary artery bypass grafting using bilateral internal thoracic arteries in patients with diabetes and obesity: A systematic review and meta-analysis. Int J Cardiol Heart Vasc 2023;47:101235. [Crossref] [PubMed]
- Formica F, Gallingani A, Tuttolomondo D, et al. Very Long-term Outcome of Bilateral Internal Thoracic Artery in Diabetic Patients: A Systematic Review and Reconstructed Time-To-Event Meta-analysis. Curr Probl Cardiol 2024;49:102135. [Crossref] [PubMed]
- Benedetto U, Gaudino M, Caputo M, et al. Right internal thoracic artery versus radial artery as the second best arterial conduit: Insights from a meta-analysis of propensity-matched data on long-term survival. J Thorac Cardiovasc Surg 2016;152:1083-1091.e15. [Crossref] [PubMed]
- Benedetto U, Caputo M, Gaudino M, et al. Right internal thoracic artery or radial artery? A propensity-matched comparison on the second-best arterial conduit. J Thorac Cardiovasc Surg 2017;153:79-88.e4. [Crossref] [PubMed]
- Flather M, Dimagli A, Benedetto U, et al. Bilateral versus single internal thoracic coronary artery bypass grafting: the ART RCT. Southampton: National Institute for Health and Care Research; 2023.
- Zhu YY, Seco M, Harris SR, et al. Bilateral Versus Single Internal Mammary Artery Use in Coronary Artery Bypass Grafting: A Propensity Matched Analysis. Heart Lung Circ 2019;28:807-13. [Crossref] [PubMed]
- Writing Committee Members. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:197-215. Erratum in: J Am Coll Cardiol 2022;79:1547. [Crossref] [PubMed]
- Omer S, Cornwell LD, Rosengart TK, et al. Completeness of coronary revascularization and survival: Impact of age and off-pump surgery. J Thorac Cardiovasc Surg 2014;148:1307-1315.e1. [Crossref] [PubMed]
- Melina G, Angeloni E, Refice S, et al. Residual SYNTAX score following coronary artery bypass grafting. Eur J Cardiothorac Surg 2017;51:547-53. [Crossref] [PubMed]
- Glineur D, Etienne PY, Kuschner CE, et al. Bilateral internal mammary artery Y construct with multiple sequential grafting improves survival compared to bilateral internal mammary artery with additional vein grafts: 10-year experience at 2 different institutions†. Eur J Cardiothorac Surg 2017;51:368-75. [Crossref] [PubMed]
- Bakaeen FG, Ravichandren K, Blackstone EH, et al. Coronary Artery Target Selection and Survival After Bilateral Internal Thoracic Artery Grafting. J Am Coll Cardiol 2020;75:258-68. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Torregrossa G, et al. Multi-spectrum robotic cardiac surgery: Early outcomes. JTCVS Tech 2022;13:74-82. [Crossref] [PubMed]
- Kitahara H, Grady K, Balkhy H. Minimally invasive multivessel coronary grafting using a robotic totally endoscopic approach. Multimed Man Cardiothorac Surg 2023;
- Kikuchi K, Chen X, Mori M, et al. Perioperative outcomes of off-pump minimally invasive coronary artery bypass grafting with bilateral internal thoracic arteries under direct vision†. Interact Cardiovasc Thorac Surg 2017;24:696-701. [Crossref] [PubMed]
- Nambiar P, Kumar S, Mittal CM, et al. Outcomes of Bilateral Internal Thoracic Arteries in Minimally Invasive Coronary Artery Bypass Grafting With Analogy to the SYNTAX Trial. Innovations (Phila) 2019;14:227-35. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Sgouropoulou S, et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J Thorac Cardiovasc Surg 2021;162:1109-1119.e4. [Crossref] [PubMed]
- Kikuchi K, Mori M. Minimally invasive coronary artery bypass grafting: a systematic review. Asian Cardiovasc Thorac Ann 2017;25:364-70. [Crossref] [PubMed]
- Masroor M, Zhou K, Chen C, et al. All we need to know about internal thoracic artery harvesting and preparation for myocardial revascularization: a systematic review. J Cardiothorac Surg 2021;16:354. [Crossref] [PubMed]
- Shafiq A, Maniya MT, Duhan S, et al. Skeletonized versus Pedicled harvesting of internal mammary artery: A systematic review and Meta-analysis. Curr Probl Cardiol 2024;49:102160. [Crossref] [PubMed]
- Hu X, Zhao Q. Skeletonized internal thoracic artery harvest improves prognosis in high-risk population after coronary artery bypass surgery for good quality grafts. Ann Thorac Surg 2011;92:48-58. [Crossref] [PubMed]
- Bakaeen FG, Ghandour H, Ravichandren K, et al. Right Internal Thoracic Artery Patency Is Affected More by Target Choice Than Conduit Configuration. Ann Thorac Surg 2022;114:458-66. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit--5,766 patients and 991 angiograms. Ann Thorac Surg 2011;92:9-15; discussion 15-7. [Crossref] [PubMed]
- Chow MS, Sim E, Orszulak TA, et al. Patency of internal thoracic artery grafts: comparison of right versus left and importance of vessel grafted. Circulation 1994;90:II129-32.
- Balkhy HH, Nathan S, Arnsdorf SE, et al. Right Internal Mammary Artery Use in 140 Robotic Totally Endoscopic Coronary Bypass Cases: Toward Multiarterial Grafting. Innovations (Phila) 2017;12:9-14. [Crossref] [PubMed]
- Nisivaco S, Kitahara H, Abutaleb A, et al. Robotic Totally Endoscopic Coronary Bypass to the Left Anterior Descending Artery: Left Versus Right Internal Thoracic Artery Grafts. J Surg Res 2023;291:139-50. [Crossref] [PubMed]







