Issues and considerations in perioperative management of robotic coronary bypass grafting
Introduction
Millions of people worldwide are affected by coronary artery disease, which remains a leading cause of morbidity and mortality (1). Coronary artery bypass surgery, which was first introduced in 1968, has been the gold standard of care for treating multi-vessel coronary artery disease due to the known benefits and advantages of the left internal mammary artery-left anterior descending (LIMA-LAD) graft (2-7). Traditionally, this operation has been performed through a median sternotomy, however, recent trends towards endoscopic and less invasive approaches in other surgical specialties have led to the adoption of minimally invasive approaches to address coronary artery disease (2,3).
The advantages of minimally invasive coronary artery bypass graft (CABG) surgery over conventional CABG include shorter recovery time, overall reduction in morbidity, fewer blood transfusions, greater patient satisfaction, shorter hospital stay, and earlier return to work. These advantages have been well established (8). These minimally invasive approaches utilize alternative sternal-sparing incisions to access the heart. The umbrella term “robotic CABG” encompasses a wide array of utilization of the robot during coronary artery revascularization, ranging from harvesting the internal thoracic arteries (ITAs) to preforming the coronary anastomoses. The perioperative management of patients undergoing robotic CABG is instrumental in the success of the operation and can be divided into preoperative and postoperative care, which includes meticulous preoperative workup for patient selection and protocolized postoperative care.
Preoperative considerations
Preoperative considerations for patients who will undergo robotic CABG includes factors that are related to the patient’s past medical history and current anatomy. Pre-habilitation and rehabilitation after surgery are based on each patient’s specific past medical history. Other factors such as smoking status and cessation, exercise tolerance and limitations, diabetic control, and weight optimization, may guide specialized referrals to dieticians or glycemic control teams to optimize the patient prior to surgery. Elderly patients may also have geriatric needs that require specific referrals.
Another aspect of the preoperative workup is the physical examination and specific imaging. Various factors that can be evaluated on physical exam, such as the external chest wall anatomy, subcutaneous tissue burden, and overall size of the thorax, are aspects that can be evaluated preoperatively and can influence the position of the robotic arms and thus affect the operation (9). Patient factors that affect whether a patient can tolerate single lung ventilation, such as chronic obstructive pulmonary disease, pulmonary hypertension, and other co-morbidities, are information that will be extracted after a thorough history (9). Pulmonary function test is another example of a necessary test that is required to be performed prior to surgery to assess whether the patient will be able to tolerate single-lung ventilation.
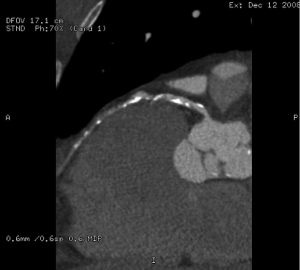
After a thorough history and physical exam, the next most important step in the workup of a patient prior to undergoing robotic CABG is preoperative imaging of the chest. A simple chest X-ray [anteroposterior (AP) and lateral view] is obtained; however this does not provide all the detailed information that is required prior to surgery. A more detailed imaging modality would be a preoperative computed tomography (CT) scan of the heart/chest, abdomen, and pelvis. This is crucial not only to assess the thoracic anatomy, but also to assess the peripheral vasculature in the event that the patient needs to be placed on cardiopulmonary bypass via the peripheral arteries during surgical revascularization. A complete evaluation of the intrathoracic spaces and analyzing the anatomy prior to the surgery increases the chances of success, avoids complications, and minimizes conversions. Evidence of lung disease on the chest CT scan with signs of obstructive lung disease should also be considered as an indication of the patient not being able to tolerate single lung ventilation and a relative contraindication for minimally invasive surgical approach to revascularization. Identification of the left anterior descending (LAD) coronary artery on preoperative imaging is important to assess the location and course of the artery, specific anatomic considerations such as lateral displacement of the LAD, and extent of the calcifications. Specific features that are crucial to identify are intra-myocardial or adipose location of the LAD and other coronary arteries, and extent of pericardial fat (Figure 1). Furthermore, evidence of chronic total occlusion (CTO) of the LAD with poor LAD target distal to the occlusion on the CT heart may also be a contraindication to minimally invasive surgical revascularization. Any of the above-mentioned features would make the surgical revascularization challenging and may be considered a relative contraindication to performing the coronary artery revascularization with the assistance of the robot as it increases the difficulty in isolating the coronary vessels and performing the anastomosis. In addition, the CT scan will provide further information regarding the anatomy of the left internal thoracic artery (LITA). Information such as the course, size, and patency of the LITA at the take off from the subclavian artery and ruling out occlusion from plaque within the subclavian artery is crucial in planning the minimally invasive surgical revascularization.
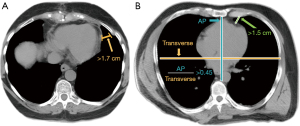
As previously demonstrated in the paper by Anderson et al., information about the thoracic cavity dimensions is helpful and highlights that a distance of >1.7 cm is necessary between the chest wall and the mediastinum at the camera port insertion site in order to accommodate the endoscopic port for the insertion of the endoscope (Figure 2) (4). A distance <1.7 cm compromises the endoscope and other instrument maneuverability and increases the likelihood of possible conversation to non-robotic approach or median sternotomy. Furthermore, knowing the position and axis of the heart with an ideal ratio of AP distance to transverse distance >0.45, is also important to not compromise robotic instrument maneuverability and the appropriate chest cavity (Figure 2) (4).
Moreover, preoperative cardiac catheterization must be reviewed to determine suitability of the patient for robotic CABG based on the location of the coronary artery disease and the distal targets. This can be reviewed with the heart team, which includes a cardiologist, an anesthesiologist, and a cardiac surgeon, to decide if the patient would be a candidate for robotic assisted revascularization and possible hybrid revascularization. Evidence of moderate to severe pulmonary hypertension may be a relative contraindication as patients with moderate or severe pulmonary hypertension will have a further increase in their pulmonary arterial pressures during single lung ventilation, which could result in acute right ventricular dysfunction and hemodynamic compromise.
The last preoperative consideration for patient selection is a holistic discussion with the patient and their family that encompasses expectation setting in terms of having the most benefit from the minimally invasive operation with arrangement for early discharge from the hospital. Shared decision making in conjunction with a collaborative approach with other members of the medical team such as anesthesiologists, allows for the selection of suitable patients by the surgeon and anesthesiologists based on grounds of compatible coronary anatomy for minimally invasive coronary artery revascularization. Careful selection of patients and a team-based approach is very important. The importance of good teamwork with experienced anesthesiologists and nurses cannot be emphasized enough.
In summary, there are physical features that can be discovered preoperatively with appropriate imaging that could be a relative contraindication for the patient to undergo robotic CABG. Specific characteristics of the LAD or other coronary vessels, such as intramyocardial location, or lack of viable targets for bypass due to size or location of the distal LAD or the other coronary vessels, all increase the potential post operative complications, risk of morbidity, and possible need for conversion to a traditional sternotomy. Inadequate space in the thorax that limits the movement of the robotic instruments also increases the chances of not being successful performing a minimally invasive approach and possible need for conversion to median sternotomy, and should be thoroughly analyzed preoperatively. Any history of prior chest surgeries or radiation to the chest will increase the chance of adhesions and could be a challenge or contraindication to undergo robotic CABG. Although no imaging can discover the presence of adhesions, it would be important to be aware of the possibility based on the patient’s history. In addition to the preoperative pulmonary function tests that would indicate the inability of the patient to tolerate single lung ventilation, findings on CT scan showing evidence of obstructive lung disease should also be considered as an indication of the same, and are an additional relative contraindication for robotic CABG. Thus, the first step when performing a robotic CABG is appropriate patient selection which is of paramount importance and is augmented by a thorough preoperative work up with physical examination and specific imaging.
Postoperative considerations
After the successful completion of a minimally invasive robotic surgical revascularization, a standardized fast track postoperative protocol is implemented. This management strategy includes appropriate multi-modal pain control with utilization of non-steroidal anti-inflammatory medications and peri-operative nerve blocks, while minimizing narcotics as much as possible. This allows the patient to be extubated intraoperatively or within 6 hours of leaving the operating room in order to advance their care and subsequently eliminating or decreasing their time in the intensive care unit (ICU). The overall goal of following a standardized fast track protocol is to decrease hospital length of stay, improve patient satisfaction, and reduce the overall costs and resources utilized by the institution, all while maintaining excellent clinical outcomes.
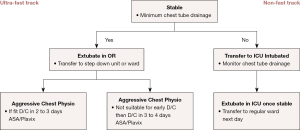
Fast track protocols have been implemented in cardiac surgery since the early 1990s and incorporate early extubation with lower narcotic doses to reduce post operative respiratory complications and have been shown to be safe, efficient, and cost beneficial by reducing the hospital length of stay (8,10). Patients will benefit the most from a minimally invasive procedure if they undergo an enhanced recovery after surgery by minimizing the length of stay in the ICU, or avoiding the ICU altogether, which is considered the ultra-fast track protocol (8). The ultra-fast track protocol, which is applicable to a select group of patients, reduces patient morbidity and decreases the costs accrued by the hospital for ICU stays (8). To be eligible for the fast track or ultra-fast track postoperative protocol, the patient must be hemodynamically stable with minimal chest tube output at the conclusion of the case (Figure 3). However, the preoperative status of the patient is just as important in the postoperative recovery phase, as critical preoperative status has been found to be a significant predictor of failure of the fast-track protocol.
Ultimately, utilizing minimally invasive techniques with the robot for coronary artery revascularization avoids the morbidity associated with median sternotomy and allows for better visualization over previous endoscopic approaches due to robotic platforms providing three-dimensional (3D) vision, magnification, and precise movements (2,3,11). Studies have shown that robotic CABG is safe and effective with reported postoperative patency rates of 97.4% which is comparable to previous studies demonstrating rates of 96.3% and 96.6% (3,12,13). The paper by Giambruno et al., which is an 18-year single center experience of patients undergoing robotic-assisted CABG surgery, found that the average length of stay in the ICU was 1.2±1.4 days and the average length of stay in the hospital was 4.8±2.9 days. This was accompanied by low postoperative complications devoid of renal or respiratory failure (3). The same study also reported perioperative myocardial infarction occurring in only 1% of patients, which was similar to other published studies (3,14,15).
However, despite favorable and comparable short- and long-term outcomes in regards to overall perioperative morality, LITA patency, re-exploration rate, and postoperative myocardial infarction rate compared to the traditional sternotomy approach for CABG, there has been a slow adoption of robotic CABG (16,17). This may be in part due to the higher costs associated with robotic technology, accessibility to robotic technology, and the learning curve that needs to be overcome.
It remains true that experienced surgeons, dedicated robotic staff, and established protocols for perioperative and postoperative care are prerequisites for a safe and successful robotic CABG surgery program (18). The paper by Xue et al. details the tools, collaboration, and institutional support that is required to establish a successful and efficient robot-assisted mitral valve surgery program, which can be extrapolated into developing a similar program for robotic CABG surgery (18). Rodriguez et al. also emphasizes that surgeons need to be well versed in not only the traditional approach, but also other minimally invasive approaches on and off pump prior to taking on the robot-assisted approach to coronary artery revascularization (19).
Conclusions
As robotic CABG surgery continues to grow, there are important aspects of perioperative management that will augment the success of the operation, from an extensive preoperative workup to protocolized postoperative care. It is imperative that the surgeon and operating team be well versed in traditional and minimally invasive coronary revascularization as the experience of the surgeon is a key factor in the successful outcome of robotic coronary revascularization given its challenging nature and steep learning curve.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: B.K. is a consultant with Medtronic, Johnson and Johnson, Corcym, and Abbott. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e18-e114. [Crossref] [PubMed]
- Atluri P, Kozin ED, Hiesinger W, et al. Off-pump, minimally invasive and robotic coronary revascularization yield improved outcomes over traditional on-pump CABG. Int J Med Robot 2009;5:1-12. [Crossref] [PubMed]
- Giambruno V, Chu MW, Fox S, et al. Robotic-assisted coronary artery bypass surgery: an 18-year single-centre experience. Int J Med Robot 2018;14:e1891. [Crossref] [PubMed]
- Anderson D, Chen S, Southard J, et al. Multidisciplinary approach to coronary artery revascularization: Optimal strategy for high-risk patients. J Card Surg 2022;37:2900-2. [Crossref] [PubMed]
- Harskamp RE, Vassiliades TA, Mehta RH, et al. Comparative Effectiveness of Hybrid Coronary Revascularization vs Coronary Artery Bypass Grafting. J Am Coll Surg 2015;221:326-34.e1. [Crossref] [PubMed]
- Panoulas VF, Colombo A, Margonato A, et al. Hybrid Coronary Revascularization: Promising, But Yet to Take Off. J Am Coll Cardiol 2015;65:85-97. [Crossref] [PubMed]
- Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996;335:217-25. [Crossref] [PubMed]
- Bainbridge D, Cheng D. Current evidence on fast track cardiac recovery management. Eur Heart J Suppl 2017;19:A3-7.
- Sellke FW, Ruel M. editors. Atlas of Cardiac Surgical Techniques. 2nd edition. Elsevier; 2019.
- Engelman RM, Rousou JA, Flack JE 3rd, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg 1994;58:1742-6. [Crossref] [PubMed]
- Woo YJ. Robotic cardiac surgery. Int J Med Robot 2006;2:225-32. [Crossref] [PubMed]
- Halkos ME, Liberman HA, Devireddy C, et al. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;147:179-85. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced “robotic” cardiac surgery: Experience in 148 patients. J Thorac Cardiovasc Surg 2001;121:842-53. [Crossref] [PubMed]
- Bachinsky WB, Abdelsalam M, Boga G, et al. Comparative study of same sitting hybrid coronary artery revascularization versus off-pump coronary artery bypass in multivessel coronary artery disease. J Interv Cardiol 2012;25:460-8. [Crossref] [PubMed]
- Poston RS, Tran R, Collins M, et al. Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Ann Surg 2008;248:638-46. [Crossref] [PubMed]
- Jonsson A, Binongo J, Patel P, et al. Mastering the Learning Curve for Robotic-Assisted Coronary Artery Bypass Surgery. Ann Thorac Surg 2023;115:1118-25. [Crossref] [PubMed]
- Whellan DJ, McCarey MM, Taylor BS, et al. Trends in Robotic-Assisted Coronary Artery Bypass Grafts: A Study of The Society of Thoracic Surgeons Adult Cardiac Surgery Database, 2006 to 2012. Ann Thorac Surg 2016;102:140-6. [Crossref] [PubMed]
- Xue A, Chen S, Ranade A, et al. How to implement a clinical robotic mitral valve surgery program. Ann Cardiothorac Surg 2022;11:504-9. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J Thorac Cardiovasc Surg 2016;152:9-13. [Crossref] [PubMed]






