Aortic annular enlargement with Y-incision/rectangular patch
Introduction
The mean aortic annulus in the United States is 23 for men and 21 for women (1). This is why the most commonly used prosthetic aortic valve is size 21–23 in all the clinical trials and large series of aortic valve replacement (AVR). The labeling of the size of the prosthetic valve is based on the inner diameter of the metal frame, which is deceiving. However, the inner diameter of the opening of the cusps (not the metal frame) is 5–7 mm smaller than the labeled valve size after implantation (2). Therefore, the need for a reproducible, safe, and more effective aortic root enlargement is critical in surgical aortic valve replacement (SAVR) to prevent prosthesis-patient mismatch (PPM). Since we developed Y-incision aortic annular enlargement (AAE) in 2020, this technique has been adopted by many institutions all over the world and proved to be reproducible. At the University of Michigan, every AVR is defaulted to have AAE, and the rate of AAE has risen to 50–60% of all AVRs (3). We have made minor modifications (4) since the Y-incision was developed, but the key steps remain the same. Herein, we discuss the step-by-step approach of the Y-incision AAE with detailed illustrations. This chapter is an indissociable complement to the operative video in Masters of Cardiothoracic Surgery in this issue of ACS (4).
Operative techniques
Preparation
We routinely obtain a transthoracic echocardiogram and a left heart catheterization for every patient to evaluate the function of the valves and the ventricles. We plan to do AAE for every patient unless we can place the largest size bioprosthetic valve (size 29) or a mechanical valve (size 27).
Exposition
Median sternotomy with aortic cannulation at the distal ascending/proximal arch. Myocardial protection is achieved through antegrade (first dose) and retrograde Buckberg cardioplegia afterwards every 20 minutes. Ice slush was applied on the anterior surface of right ventricle to maintain myocardial temperature below 10 ℃. We cool the body temperature to 34 ℃ for isolated AVR.
Operation
- The aortic cannulation should be at the innominate artery level to leave enough length for the enlargement of the proximal ascending aorta and aortotomy closure with “Roof technique” (5). A partial or complete transverse aortotomy approximately 2–2.5 cm above the sinotubular junction (STJ) anteriorly and 1 cm above the STJ posteriorly is made. A more distal aortotomy anteriorly will allow for the struts of the upsized valve to lay more proximal in the ascending aorta and not protrude at the aortotomy. In reoperative SAVR, partial aortotomy can be used, especially when the medial side of the proximal ascending aorta is adhered to the main pulmonary artery.
- The aortic root above the left and right atrium is dissected out to the level of the dome of the left atrium and the nadir of the non-coronary sinus. By doing this, one can avoid cutting into the left or right atria or aberrant circumflex artery (Figure 1).
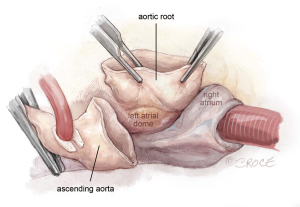 Figure 1 Aortotomy and aortic root dissection. The transverse aortotomy can be complete or partial. We use complete aortotomy for first time AVR, and partial in reoperative AVR when the medial side of the proximal ascending aorta is tightly adherent to the main pulmonary artery. The aortotomy should be 2–2.5 cm distal from the STJ. This allows the struts of upsized large valves, most frequently size 29, to avoid protruding from the transverse aortotomy, and instead be proximal to the aortotomy. The aortic root above the left and right atrium is dissected out to the level of the dome of the left atrium and the nadir of the non-coronary sinus. AVR, aortic valve replacement; STJ, sinotubular junction.
Figure 1 Aortotomy and aortic root dissection. The transverse aortotomy can be complete or partial. We use complete aortotomy for first time AVR, and partial in reoperative AVR when the medial side of the proximal ascending aorta is tightly adherent to the main pulmonary artery. The aortotomy should be 2–2.5 cm distal from the STJ. This allows the struts of upsized large valves, most frequently size 29, to avoid protruding from the transverse aortotomy, and instead be proximal to the aortotomy. The aortic root above the left and right atrium is dissected out to the level of the dome of the left atrium and the nadir of the non-coronary sinus. AVR, aortic valve replacement; STJ, sinotubular junction. - The valve is examined, and the position of the left and right coronary ostia are identified. In some cases, the coronary ostia can be low and close to the aortic annulus. In bicuspid aortic valve, the left coronary ostium can be close to the left-non commissure. These conditions are not contraindications of Y-incision annular enlargement. The stenotic aortic valve is excised, and the annulus debrided of calcium. In reoperative SAVR, the previous prosthetic should be completely removed, including all the pledgets. All the pannus underneath the prosthetic valve should be resected to restore the normal size of the basal ring and left ventricular outflow tract (LVOT).
- The Y-incision AAE proceeds with an incision of the left-non commissure from the aortotomy into the aorto-mitral curtain. The incision is extended in a “Y” fashion into the aorto-mitral curtain parallel to and below the aortic annulus, undermining the left and non-coronary annulus to their respective nadir by cutting into the left and right fibrous trigones (Figure 2). It is important to note that the incision should not cut through the trigones completely, instead it should stop 2–3 mm proximal to the myocardium on the left side and to the membranous septum on the non-coronary sinus side. This maneuver widely opens the aortic root. A common pitfall is when the Y-incision is not wide enough into the fibrous trigones, the patch ends up too small and the enlargement is limited to 1–2 valve sizes. The normal distance between the nadirs of left and non-coronary sinuses is about 3 cm, which should be the width of the Y-incision. If the left or right fibrous trigone is divided completely or near completely inadvertently, we recommend repairing the fibrous trigones with 4-0 PROLENE (Ethicon, Somerville, NJ, USA) before sewing the Daron patch to the aortic root.
In the reoperative AVR, the prior prosthesis should be completely removed. The pledgets and pannus under the valve should be resected to restore the normal diameter of the basal ring and LVOT. The Y-incision should be made underneath the aortic annulus through the space where the previous pledgets were placed, which is usually lower than one thinks.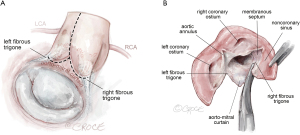 Figure 2 The Y-incision. The incision is extended in a “Y” fashion into the aortomitral curtain parallel to the aortic annulus undermining the left and noncoronary annulus to their respective nadir by cutting into the left and right fibrous trigones (A,B). The Y-incision should be into the left and right fibrous trigones but not through them, to allow for maximum enlargement of the aortic annulus and root. If the Y-incision is not wide enough into the fibrous trigones, the patch will be smaller, and the enlargement is limited to two valve sizes. The Y-incision should stop about 2–3 mm away from the myocardium next to the left fibrous trigone and membranous septum, and adjacent to the right fibrous trigone. LCA, left coronary artery; RCA, right coronary artery.
Figure 2 The Y-incision. The incision is extended in a “Y” fashion into the aortomitral curtain parallel to the aortic annulus undermining the left and noncoronary annulus to their respective nadir by cutting into the left and right fibrous trigones (A,B). The Y-incision should be into the left and right fibrous trigones but not through them, to allow for maximum enlargement of the aortic annulus and root. If the Y-incision is not wide enough into the fibrous trigones, the patch will be smaller, and the enlargement is limited to two valve sizes. The Y-incision should stop about 2–3 mm away from the myocardium next to the left fibrous trigone and membranous septum, and adjacent to the right fibrous trigone. LCA, left coronary artery; RCA, right coronary artery. - A 2×3 inches (5×7.5 cm) rectangular-shaped Hemashield Dacron patch (Boston Scientific Corp, Natick, MA, USA) is trimmed in width 5 mm greater than the distance between the two cusp nadirs on each end. We routinely use a 1.5 inches (3.75 cm) or wider patch for every patient. The wider patch allows for more extensive enlargement of the aorto-mitral curtain and the implantation of larger valves. The patch can be further trimmed if it is too wide when sewn to the aorto-mitral curtain.
- The patch is sewn to the aorto-mitral curtain from the left to right fibrous trigone with running 4-0 PROLENE suture. At the left and right fibrous trigones, the suture line is transitioned to the undermined aortic annulus at the nadir of both the left and non-coronary sinuses, sutured along the longitudinal length of the patch up to the level of the transverse aortotomy incision, and secured with additional 4-0 PROLENE suture (Figure 3). This suture line must be watertight. Any “dog ear” or possible bleeding spot should be repaired with an interrupted 4-0 or 5-0 PROLENE from inside to outside through the patch and aorto-mitral curtain. In the reoperative AVR, the aorto-mitral curtain is frequently damaged after the prior prosthetic valve is removed. The patch was usually sewn to the remnant of the aorto-mitral curtain and the anterior mitral annulus.
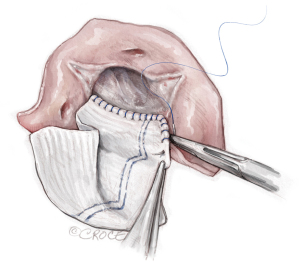 Figure 3 The patch. A 2×3 inches (5×7.5 cm) rectangular-shaped Hemashield Dacron patch (Boston Scientific Corp, Natick, MA, USA) is trimmed in width 5 mm greater than the distance between the two cusp nadirs on each end. This patch is sewn to the aortomitral curtain/mitral annulus from the left fibrous trigone to the right fibrous trigone with running 4-0 PROLENE suture (Ethicon, Somerville, NJ, USA). The suture line is transitioned to the aortic annulus at the nadir of both the left and non-coronary sinuses, sutured along the longitudinal length of the patch up to the level of the transverse aortotomy incision, and secured with additional 4-0 PROLENE suture. We routinely use a 3.75–4 cm (>1.5 inches) or wider Hemashield Dacron patch for every patient. The wider patch allows for more extensive enlargement of the aortomitral curtain and for the implantation of larger valves.
Figure 3 The patch. A 2×3 inches (5×7.5 cm) rectangular-shaped Hemashield Dacron patch (Boston Scientific Corp, Natick, MA, USA) is trimmed in width 5 mm greater than the distance between the two cusp nadirs on each end. This patch is sewn to the aortomitral curtain/mitral annulus from the left fibrous trigone to the right fibrous trigone with running 4-0 PROLENE suture (Ethicon, Somerville, NJ, USA). The suture line is transitioned to the aortic annulus at the nadir of both the left and non-coronary sinuses, sutured along the longitudinal length of the patch up to the level of the transverse aortotomy incision, and secured with additional 4-0 PROLENE suture. We routinely use a 3.75–4 cm (>1.5 inches) or wider Hemashield Dacron patch for every patient. The wider patch allows for more extensive enlargement of the aortomitral curtain and for the implantation of larger valves. - The enlarged aortic annulus/root is sized with the valve shape end of the sizer and the largest size that can touch the three nadirs of aortic annulus (left, right and non-coronary sinus) with one strut facing the left-right commissure is chosen (Figure 4). We routinely start with a valve sizer three sizes greater than the native aortic annular diameter, for example, if the annulus was 19 mm, we start with a size 25 sizer. If either one of the coronary ostia arises low (≤5 mm from the nadir), we recommend downsizing the prosthetic valve by one size. Once the largest sizer is decided, the position of the sizer on the patch is marked for valve sutures, with the patch stretched upward to guide the placement of valve sutures (Figure 5).
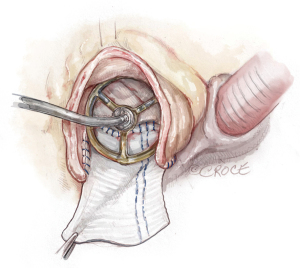 Figure 4 Sizing. The valve sizer is placed in the enlarged root touching all three nadirs of aortic annulus to determine the size of prosthesis. We routinely start with a valve sizer three sizes larger than the native annulus. The height of the divided left-non commissure is marked on the patch where the highest suture should be placed, and the upsized valve sizer is traced onto the patch. While the patch is pulled upward, the chosen bioprosthetic valve is placed into the enlarged aortic root with one strut facing the left-right commissure to confirm the marking of the valve sutures on the patch matching the sewing ring of the bioprosthesis, and the two coronary ostia not obstructed. If either one of the coronary ostia arose low (≤5 mm from the nadir), we recommend downsizing the prosthetic valve by one size.
Figure 4 Sizing. The valve sizer is placed in the enlarged root touching all three nadirs of aortic annulus to determine the size of prosthesis. We routinely start with a valve sizer three sizes larger than the native annulus. The height of the divided left-non commissure is marked on the patch where the highest suture should be placed, and the upsized valve sizer is traced onto the patch. While the patch is pulled upward, the chosen bioprosthetic valve is placed into the enlarged aortic root with one strut facing the left-right commissure to confirm the marking of the valve sutures on the patch matching the sewing ring of the bioprosthesis, and the two coronary ostia not obstructed. If either one of the coronary ostia arose low (≤5 mm from the nadir), we recommend downsizing the prosthetic valve by one size.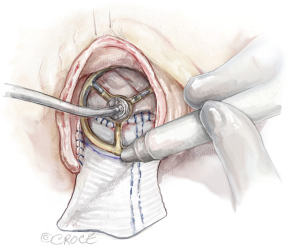 Figure 5 Marking. With one strut facing the L-R commissure, the largest sizer that was able to touch the three nadirs, is traced on the patch for the placement of the valve sutures. The tracing on the patch should be a dome shape with the top at the same level of the divided left-non commissure by the Y-incision. L-R, left-right.
Figure 5 Marking. With one strut facing the L-R commissure, the largest sizer that was able to touch the three nadirs, is traced on the patch for the placement of the valve sutures. The tracing on the patch should be a dome shape with the top at the same level of the divided left-non commissure by the Y-incision. L-R, left-right. - Once the size of the prosthetic valve is decided and the prosthesis is available, we place the prosthetic valve into the enlarged aortic root with one strut facing the left-right commissure, confirm the two coronary ostia are not obstructed and the marking for the valve sutures matches the sewing ring of the prosthetic valve. The highest position of the valve sutures on the patch should be at the height of the divided left-non commissure, and the same height of the valve sutures at the left-right and right-non commissures (Figure 6).
 Figure 6 The valve sutures. Non-pledgetted 2-0 ETHIBOND sutures (Ethicon, Somerville, NJ, USA) are placed along the native aortic annulus in a non-everting fashion and along the marking on the patch in an inside-outside-inside fashion. The highest position of the valve sutures on the patch should be at the height of the divided left-non commissure, and the same height of the valve sutures at the left-right and right-non commissures, but not necessarily in the middle of the patch. Six transitional sutures highlighted in bright blue are passed through the native aortic annular tissue and patch both, transitioning from valve suture on the native aortic annulus only, to on the patch only. The middle transitional sutures are almost vertical to bring the valve sutures up gradually to the highest position on the patch.
Figure 6 The valve sutures. Non-pledgetted 2-0 ETHIBOND sutures (Ethicon, Somerville, NJ, USA) are placed along the native aortic annulus in a non-everting fashion and along the marking on the patch in an inside-outside-inside fashion. The highest position of the valve sutures on the patch should be at the height of the divided left-non commissure, and the same height of the valve sutures at the left-right and right-non commissures, but not necessarily in the middle of the patch. Six transitional sutures highlighted in bright blue are passed through the native aortic annular tissue and patch both, transitioning from valve suture on the native aortic annulus only, to on the patch only. The middle transitional sutures are almost vertical to bring the valve sutures up gradually to the highest position on the patch. - Non-pledgetted 2-0 ETHIBOND sutures (Ethicon, Somerville, NJ, USA) are placed along the native aortic annulus in a non-everting fashion for bioprosthetic valves and Top Hat CarboMedics mechanical valves (CarboMedics, Austin, TX, USA) and from inside-outside-inside on the patch (Figure 6). For St. Jude Medical mechanical valves (St. Jude Medical Inc., Minneapolis, MN, USA), we use pledgetted everting sutures. The valve suture line should be marked over the sizer on the patch in a dome shape (Figure 6). It is worth reiterating that the highest position of the valve sutures on the patch should be at the height of the divided left-non commissure, and the same height of the valve sutures at the left-right and right-non commissures, but not necessarily in the middle of the patch. If the valve sutures on the patch are placed too low (significantly lower than the height of the divided left-non commissure), the upsizing is limited to one-to-two valve sizes; otherwise, the large valve could pinch the ostium of the right coronary since the prosthetic valve pushes anteriorly and cephalad.
- The bioprosthesis is placed with one strut facing the left-right commissure, and the valve sutures are divided by three and evenly distributed to the sewing ring of three cusps of the prosthetic valve (Figure 7). It is critical to start with passing the valve sutures at the left-right commissure through the sewing ring on each side of the strut of the prosthetic valve, so that the strut is perfectly facing the left-right commissure and the coronary ostia are on each side of the strut equally. In patients with a bicuspid aortic valve, Sievers type 0, and no left-right commissure, the strut of the prosthetic valve is placed at the middle point between the two coronary ostia to set the coronary ostia equally on each side of the strut. All valve sutures should be divided into thirds and placed evenly as normally done in SAVR. The other two struts will sit accordingly based on the valve sutures, most frequently mildly rotated clockwise, without interfering with the coronary flow. For mechanical valves, we place the discs parallel to the muscular ventricular septum (6,7) with one pivot guard at the left-right commissure and coronary ostia on each side of the disc (6).
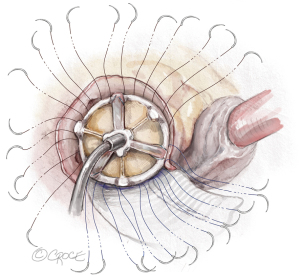 Figure 7 Seating of the prosthetic valve. The upsized prosthetic valve is placed with one strut facing the left-right commissure with the coronary ostium on each side of the strut equally. The valve sutures are divided by three and evenly distributed to the sewing ring of the three cusps of the bioprosthetic valve.
Figure 7 Seating of the prosthetic valve. The upsized prosthetic valve is placed with one strut facing the left-right commissure with the coronary ostium on each side of the strut equally. The valve sutures are divided by three and evenly distributed to the sewing ring of the three cusps of the bioprosthetic valve. - The sutures at the nadirs of the non-coronary and left-coronary sinuses are the lowest point of the aortic annulus. We tie three pairs of valve sutures at the nadir of the non-coronary sinus first, then at the left-coronary sinus to seat the valve and prevent paravalvular leak (Figure 8). We then tie the valve sutures around the right coronary sinus and sutures on the patch last. A portion of the patch lays beneath the prosthetic valve thereby enlarging the aorto-mitral curtain to enlarge the surgical aortic annulus/aortic root. Both left and right coronary ostia are confirmed to be open (Figure 9). Since the root was extensively enlarged, even with a large prosthetic valve, the valve-coronary-distance was 5–7 mm which was a good setup for future valve-in-valve TAVR.
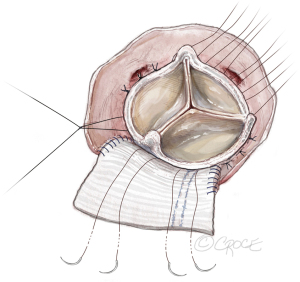 Figure 8 Tying the valve sutures. The sutures around the nadirs of the non-coronary and left coronary sinuses (three pairs of sutures for each sinus), are the lowest point of aortic annulus and are tied first. Most frequently, the right-non strut is located on the right to the right-non commissure and left-non strut is located at the center or left to the center of the patch.
Figure 8 Tying the valve sutures. The sutures around the nadirs of the non-coronary and left coronary sinuses (three pairs of sutures for each sinus), are the lowest point of aortic annulus and are tied first. Most frequently, the right-non strut is located on the right to the right-non commissure and left-non strut is located at the center or left to the center of the patch.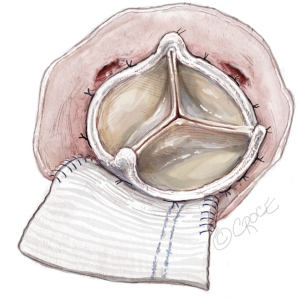 Figure 9 Checking the coronary ostia and gap between the sewing ring and annulus. Confirming the left (upper) and right (lower) coronary ostia are not blocked by the prosthetic valve and there is no gap between the sewing ring and the aortic annulus with a large nerve hook. Sometimes, the coronary ostium (most frequently the left) is below the valve but above the sewing ring. There is frequently large space between the coronary ostium and prosthetic valve. The coronary perfusion would be fine. If the right coronary ostium is pinched by the prosthetic valve, the valve sutures on the patch could be too low, far below the level of the divided left-non commissure. Surgeons could redo the valve sutures on the patch by placing them high with the highest at the level of divided left-non commissure. If the valve sutures are high enough and the right coronary ostium is still covered by the sewing ring of the prosthetic valve, then the surgeon needs to downsize the valve by one valve size.
Figure 9 Checking the coronary ostia and gap between the sewing ring and annulus. Confirming the left (upper) and right (lower) coronary ostia are not blocked by the prosthetic valve and there is no gap between the sewing ring and the aortic annulus with a large nerve hook. Sometimes, the coronary ostium (most frequently the left) is below the valve but above the sewing ring. There is frequently large space between the coronary ostium and prosthetic valve. The coronary perfusion would be fine. If the right coronary ostium is pinched by the prosthetic valve, the valve sutures on the patch could be too low, far below the level of the divided left-non commissure. Surgeons could redo the valve sutures on the patch by placing them high with the highest at the level of divided left-non commissure. If the valve sutures are high enough and the right coronary ostium is still covered by the sewing ring of the prosthetic valve, then the surgeon needs to downsize the valve by one valve size. - Closing the aortotomy with “Roof” technique (5): a longitudinal aortotomy is made at the posterior aspect of the proximal ascending aorta (Figure 10). The distal end of the rectangular patch is trimmed to a generous triangular shape, with the tip of the triangle 2 cm above the level of the posterior strut. The aortotomy is closed with 4-0 PROLENE by incorporating the triangular shaped end of the patch into the longitudinal aortotomy as is previously described in the “Roof” technique (5). The aortotomy closure could start from the native aorta at the pulmonary artery side, or from the apex of longitudinal aorta sewn to the apex of the triangular shape end of the patch (Figure 11).
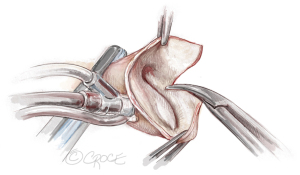 Figure 10 Longitudinal aortotomy. A 2–3 cm longitudinal aortotomy is made in the posterior proximal ascending aorta and the distal end of the rectangular patch is trimmed in a triangular shape symmetrically with the tip of the triangle 2 cm above the strut. The longitudinal aortotomy could aim slightly towards the greater curvature side of the ascending aorta to make the closure easier.
Figure 10 Longitudinal aortotomy. A 2–3 cm longitudinal aortotomy is made in the posterior proximal ascending aorta and the distal end of the rectangular patch is trimmed in a triangular shape symmetrically with the tip of the triangle 2 cm above the strut. The longitudinal aortotomy could aim slightly towards the greater curvature side of the ascending aorta to make the closure easier.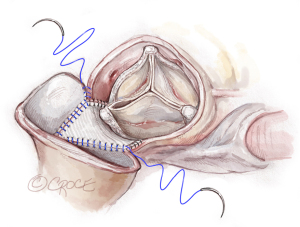 Figure 11 Aortotomy closure. The aortotomy is closed with 4-0 PROLENE by incorporating the triangular shape end of the patch into the longitudinal aortotomy as the “Roof” technique (5). The closing of the aortotomy can start from the pulmonic artery side of the transverse aortotomy by the primary surgeon or from the apex of the longitudinal aortotomy by the first assistant.
Figure 11 Aortotomy closure. The aortotomy is closed with 4-0 PROLENE by incorporating the triangular shape end of the patch into the longitudinal aortotomy as the “Roof” technique (5). The closing of the aortotomy can start from the pulmonic artery side of the transverse aortotomy by the primary surgeon or from the apex of the longitudinal aortotomy by the first assistant. - After the aorta was unclamped, the aortic root and proximal ascending aorta are enlarged extensively. The patch laid at the posterior side of the aorta. The valve sits supra-annularly and perpendicular to the blood flow, not tilted (Figure 12).
Completion
After protamine is given, frequently, no additional measure is needed apart from packing. In patients with severe coagulopathy, blood products should be given, including activated Factor VII if needed. In our 142 consecutive cases, including patients with aortic valve endocarditis, no patients needed reoperation for bleeding from the patch or aortotomy, and one patient needed reoperation for bleeding from the chest wall.
Comments
Clinical results
We have done a total of 142 Y-incision annular enlargement cases since August 2020. Two-thirds of our patients are female, >50% were obese, 1/3 were reoperations and 1/3 had multiple procedures besides AVR (including 9% planned mitral repair or replacement). The most common valve we used was size 29 valve or the largest valve size (size 27 in mechanical valves). We had one operative death who had radiation of the chest and abdomen for lymphoma and underwent AVR with AAE, mitral valve replacement, coronary artery bypass and MAZE procedure. The patient died from mesenteric ischemia on postoperative day 35. Two patients had complete heart block and permanent pacemaker (including one who had aortic valve endocarditis complicated with Gerbode fistula), and one had exacerbation of a preoperative stroke. The overall median aortic valve mean gradient was 6–7 mmHg, the median aortic root increased from 27 to 40 mm, the median STJ increased from 29 to 36 mm, and the median valve-to-coronary distance was 5–7 mm in the cohort during 2-year follow-up (2). The median left ventricular mass index regression was 41% at 12–24 months in patients with moderate/severe aortic stenosis.
Advantages
The Y-incision AAE gives the patient a prosthetic valve with an inner diameter of the opening of the cusps similar to or larger than the native annulus, avoiding the under sizing that occurs when relying on the inner diameter of the metal frame reported by the valve companies. This restores normal hemodynamics across the aortic valve completely, avoiding PPM and improving patients’ quality of life. The larger valve could improve patients’ long-term survival (8,9), and potentially improve the longevity of the prosthetic valves, since the most common mechanism of deterioration for bovine pericardial valves is calcification and stenosis. In addition, the larger valve (≥ size 25) plus enlarged aortic root and STJ provide an optimized setup for future valve-in-valve TAVR (2). The enlargement both accommodates a larger endovascular valve size and provides a safer landing zone with increased coronary ostia to leaflet length (valve-to-coronary distance). Compared to traditional techniques of AAE, such as Nicks, Manougian, or Konno procedures, Y-incision AAE does not violate any structure of the heart surrounding the aortic root, and has a much lower risk of bleeding, mitral regurgitation, complete heart block, aortic root-right ventricular outflow fistula, while being more effective at upsizing the valve sizes (2,10).
Caveats
The Y-incision AAE enlarges the surgical aortic annulus where surgeons place valve sutures to implant a large valve and enlarge the aortic annulus/root to achieve adequate valve-to-coronary distance for future valve-in-valve TAVR. This technique does not enlarge the LVOT. If there is LVOT stenosis, such as subannular web or hypertrophic obstructive cardiomyopathy (HOCM), we recommend resecting the subannular web in the LVOT or, a myectomy for HOCM as surgeons normally do, then performing the Y-incision AAE to place a large prosthetic valve in the aortic root. Bleeding is always a concern. The suture line of the patch to the aorto-mitral curtain and aortic annulus has to be watertight. Surgeons should have a low threshold to put additional interrupted repairing sutures if there is any suspicion for bleeding right after the patch is sewn to the aortic root. It is critical to place the six transitional sutures to bring the valve sutures from the nadirs up to patch (Figure 6). On the patch, the valve sutures should follow the marking of the sizer, which should be the dome shape with the top at the level of the divided left-non commissure and should not be low or close to the mitral annulus. Otherwise, a large prosthetic valve could push forward and pinch the ostium of the right coronary artery. Because of the massive enlargement of the aortic root and STJ, it could be challenging to close the transverse aortotomy. The “Roof” technique is a good solution to close the aortotomy and enlarge the proximal ascending aorta to prepare for future valve-in-valve TAVR. It is important to place the patch on the inside of the aorta when one is closing the posterior longitudinal aortotomy to prevent bleeding (Figure 11). Bleeding from the posterior longitudinal aortotomy closure can be repaired with additional 4-0 or 5-0 PROLENE with little difficulty. Some surgeons use an anterior longitudinal aortotomy and add an additional triangular patch anteriorly or use the patch all the way across a partial transverse aortotomy to close the aortotomy. We have not seen the postoperative computed tomography aortogram (CTA) of the aorta in patients treated with those techniques of aortotomy closure. Bleeding below the prosthetic valve is very difficult to repair since the valve is very large. We had one out of 142 cases complicated with intraoperative bleeding due to an injury of the dome of the left atrium from suctioning. We had to reclamp the aorta, reinforce the suture line of the patch to the aortomitral curtain with two interrupted stitches, and sew the dome of the left atrium to the patch below the prosthetic valve from inside of the aortic root to stop the bleeding. We recommend the complete transverse aortotomy plus a posterior longitudinal aortotomy of the ascending aorta [“Roof” technique (5)] because this combination of two aortotomies has produced the best alignment of ascending aorta and aortic root on postoperative CTA of the aorta (Vid. 1). A partial transverse aortotomy leaving the medial side of the aorta intact and “Roof” technique for aortotomy closure also has acceptable results, and makes the closure of the aortotomy simpler, especially in reoperative AVR. We have not seen any other intraoperative complications or needs of additional unplanned procedures, such as obstruction of coronary ostia, coronary torsion, severe mitral regurgitation or paravalvular leak. We have not had to take the prosthetic valve out and change to a smaller valve after first attempt in the 142 consecutive cases we have done.
Acknowledgments
Funding: B.Y. is supported by NIH R01HL141891 and R01HL151776.
Footnote
Conflicts of Interest: B.Y. is supported by NIH R01HL141891 and R01HL151776. B.Y. also serves as consultant to Medtronic, Edwards, and Ethicon and course director for Artivion. The author has no other conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Capps SB, Elkins RC, Fronk DM. Body surface area as a predictor of aortic and pulmonary valve diameter. J Thorac Cardiovasc Surg 2000;119:975-82. [Crossref] [PubMed]
- Yang B, Ghita C, Makkinejad A, et al. Early outcomes of the Y-incision technique to enlarge the aortic annulus 3 to 4 valve sizes. J Thorac Cardiovasc Surg 2024;167:1196-1205.e2. [Crossref] [PubMed]
- Yang B, Burris NS, Prasch P. Reply from authors: Not lemon on a stick, but crown (valve) on a head (left ventricular outflow tract). JTCVS Tech 2022;16:22-4. [Crossref] [PubMed]
- Yang B, Monaghan K, Hassler K, et al. Updates on Y-incision aortic annular enlargement. Ann Cardiothorac Surg 2024; [Crossref]
- Yang B, Naeem A, Palmer S. "Roof" technique-a modified aortotomy closure in Y-incision aortic root enlargement upsizing 3-4 valve sizes. JTCVS Tech 2022;12:33-6. [Crossref] [PubMed]
- Yang B, Naeem A A. Y Incision and Rectangular Patch to Enlarge the Aortic Annulus by Three Valve Sizes. Ann Thorac Surg 2021;112:e139-41. [Crossref] [PubMed]
- Yang B, Ghita C, Palmer S. Mechanical AVR With Y-Incision Aortic Annular Enlargement. CTSNet 2022. doi:
10.25373/ctsnet.19739392 . - Yang B. Aortic Valve Replacement vs Aortic Valve Replacement + Annular Enlargement: Apples to Oranges? Ann Thorac Surg 2024;117:479-80. [Crossref] [PubMed]
- Yang B, Makkinejad A, Fukuhara S, et al. Stentless Versus Stented Aortic Valve Replacement for Aortic Stenosis. Ann Thorac Surg 2022;114:728-34. [Crossref] [PubMed]
- Makkinejad A, Hua J, Hassler KR, et al. Comparison of the short-term outcomes between Y-incision aortic annular enlargement and traditional aortic annular enlargement techniques. Ann Cardiothorac Surg 2023; [Crossref]


















