Effectiveness of Maze IV procedure versus thoracoscopic ablation for atrial fibrillation: a propensity score-matched analysis
Introduction
The burden of disease from atrial fibrillation (AF) has been on the rise since the beginning of the 21st century. It is estimated that there are over 33 million cases of AF worldwide, with over 10 million in China alone (1,2). AF carries a significant health burden and can lead to complications such as stroke, heart failure, and sudden death (3). While surgical interventions for “lone” AF, such as the Cox-Maze IV procedure (CMP-IV) and thoracoscopic ablation (TA) procedure, boast a high success rate, the overall efficacy of AF treatment remains unsatisfactory. The quest for optimal treatment strategies in managing AF has been a subject of ongoing contention. The CMP-IV procedure, a classic treatment for AF, is highly successful, but as an open surgical procedure may potentially limit its acceptance among patients (4). In China, the use of CMP-IV to treat “lone” AF is also limited. The TA procedure, on the other hand, adopts minimally invasive technology and reduces surgical trauma. However, the simplification of the ablation route (5) prompts inquiry into whether this modification has impacted the curative efficacy of TA for AF and how it compares to CMP-IV. The lack of relevant controlled studies has made it difficult to provide an answer. In this study, data were gathered from two distinct patient groups, and we assessed the outcomes of these groups through the application of propensity score matching (PSM).
Methods
Patient population
We collected data from 459 patients with “lone” AF who underwent either CMP-IV (n=93) or TA via the left chest (n=366) between January 2015 and June 2022. To enhance the comparability of both treatment groups, we measured the baseline characteristics of all patients, including demographic characteristics such as age, gender, body mass index (BMI), and echocardiographic data containing left atrium diameters (LAD), left ventricular ejection fraction (LVEF), and left atrial appendage thrombus (LAAT). We also considered clinical characteristics including hypertension, diabetes, heart failure, coronary artery disease (CAD), previous cerebrovascular accidents, and previous catheter ablation history.
We prioritized the CMP-IV procedure for patients presenting with LAAT, previous left chest surgeries, or unsuccessful catheter ablations. Alternatively, the TA procedure was advised for individuals with significantly compromised cardiac function, or for frail and/or elderly patients. In other instances, we ensured that patients’ wishes were paramount and thoroughly honored.
Surgical method
The CMP-IV group underwent general anesthesia with endotracheal intubation, using median sternotomy or right mini-thoracotomy. After systemic heparinization, extracorporeal circulation was established through the aorta/vena cava or femoral vessels. Antegrade perfusion was achieved with cardioprotective solution, and the aortic root was used for cardiac arrest with bladder temperature of 32–35 degrees. The ablation route included the standard Maze IV route for both the left and right atrium, with all ablation lines completed using bipolar radiofrequency ablation clamps (AtriCure, Mason, OH, USA). Cryoablation or ablation pen was used to ablate the mitral valve isthmus and cavotricuspid isthmus. The left atrial appendage (LAA) was resected using a stapler, or the orifice was closed with continuous suturing from the endocardial double layer. The TA procedure via the left chest was performed as described previously (6), using three ports near the midaxillary line of the left chest wall. Three circular and three linear ablation lesions were made on the left atrium. The LAA was excluded using an Endo GIATM stapler (Covidien, Mansfield, MA, USA). The ganglion plexus (GPs) and ligament of Marshall (LOM) were ablated epicardially. If AF persisted, immediate cardioversion was performed.
Postoperative follow-up
Oral anticoagulation and antiarrhythmic drugs (AADs) were continued for 3 months post-operation. During this blank period, the onset of AF was not counted. Follow-up visits were conducted in-person or via telephone, including physical examinations, transthoracic echocardiogram, electrocardiogram, and 24 h Holter at 6- and 12-month post-ablation, and every 6 months thereafter. Holter monitoring was performed for any cardiac symptoms. Any documented episodes of atrial tachyarrhythmias lasting for 30 seconds or longer, were considered as recurrence. Head magnetic resonance imaging (MRI), cardiac computed tomography (CT), and echocardiography were performed at least once during postoperative follow-up.
Statistical analysis
Descriptive statistics were calculated to show the differences in baseline characteristics of patients in the CMP-IV and TA procedure cohorts. Categorical variables, expressed in percentages or frequencies, were analyzed using chi-squared test or Fisher’s exact test as appropriate. Continuous variables, such as age, were expressed as mean ± standard deviation and compared using the unpaired Student’s t-test. However, if continuous variable was not normally distributed, it was shown as median and interquartile range (IQR), as well as being compared using Mann-Whitney test. To reduce selection bias and expressed standardized mean difference, we performed a PSM for the patients of both groups using the R package ‘MatchIt’: 1-to-1 pairing and nearest neighbor methods, with a caliper of 0.2. The propensity score was calculated using baseline characteristics, including age, gender, BMI, duration of AF, type of AF, CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age, and sex category), LAD and LVEF. The log-rank test was performed to assess if there was a significant difference in Kaplan-Meier survival curves between the CMP-IV and TA procedure groups. We use R package “ComparisonSurv” to compare the cumulative survival rates at a fixed time point by “log-rank” statistical inference method. In all comparisons, P values <0.05 indicated statistical significance. Factors associated with recurrence were determined using univariate and multivariable Cox regression. In the matched cohort, the time of recurrence event at 1 and 3 years were assessed using a Cox proportional hazards model, and the estimates were presented as hazard ratios along with 95% confidence intervals (CIs). These statistical analyses were performed using the R language (R version 4.1.1).
Ethical review
This study has been approved by the Medical Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-D-2022-004), and informed consent was obtained from all participants.
Results
Unmatched population
A total of 459 patients were enrolled in the study, of whom 366 had undergone TA, and 93 underwent CMP-IV. The median age of TA patients was 61 years, significantly older than CMP-IV (58 years, P=0.027). There was a higher proportion of paroxysmal AF in TA group (74/366, 20.2%) than those in CMP-IV group (14/93, 15.1%). In echocardiographic data compared to the TA group, CMP-IV group were noted for larger LAD (46.9±5.8 vs. 44.1±5.0, P<0.001) and better cardiac function [LVEF 57.0% (53.0%, 61.0%) vs. 56% (51.0%, 60.0%), P=0.036]. In addition, 69.9% (65/93) of CMP-IV group had LAAT compared to only 2.2% (8/366) of patients undergoing TA. Baseline characteristics are presented in Table 1.
Table 1
| Characteristics | All included patients | PSM patients | ||||||
|---|---|---|---|---|---|---|---|---|
| TA (n=366) | CMP-IV (n=93) | P value | SMD | TA (n=87) | CMP-IV (n=87) | SMD | ||
| Age, year | 61.0 [54.3, 68.0] | 58.0 [54.0, 64.0] | 0.027 | 0.214 | 59.0 [52.0, 66.5] | 58.0 [54.0, 64.0] | 0.040 | |
| Male | 244 (66.7) | 62 (66.7) | >0.999 | <0.001 | 58 (66.7) | 58 (66.7) | <0.001 | |
| BMI, kg/m2 | 26.3±3.2 | 26.0±3.5 | 0.503 | 0.076 | 25.9±2.9 | 26.2±3.5 | 0.085 | |
| Hypertension | 239 (65.3) | 68 (73.1) | 0.191 | 0.170 | 59 (67.8) | 63 (72.4) | 0.101 | |
| Diabetes | 78 (21.3) | 19 (20.4) | 0.965 | 0.022 | 22 (25.3) | 16 (18.4) | 0.168 | |
| Previous cerebrovascular accident | 37 (10.1) | 15 (16.1) | 0.146 | 0.179 | 9 (10.3) | 15 (17.2) | 0.201 | |
| Coronary artery disease | 55 (15.0) | 9 (9.7) | 0.245 | 0.163 | 15 (17.2) | 9 (10.3) | 0.201 | |
| NYHA class III–IV | 128 (35.0) | 31 (33.3) | 0.808 | 0.035 | 24 (27.6) | 28 (32.2) | 0.101 | |
| CHA2DS2-VASc score | 2.0 [1.0, 3.0] | 2.0 [1.0, 3.0] | 0.455 | 0.097 | 2.0 [1.0, 3.0] | 2.0 [1.0, 3.0] | 0.024 | |
| Previous catheter ablation | 57 (15.6) | 21 (22.6) | 0.147 | 0.179 | 13 (14.9) | 19 (21.8) | 0.220 | |
| Non-paroxysmal AF | 292 (79.8) | 79 (84.9) | 0.527 | 0.136 | 75(86.2) | 73 (83.9) | 0.163 | |
| Duration of AF, months | 48.0 [24.0, 72.0] | 48.0 [24.0, 72.0] | 0.123 | 0.159 | 48.0 [24.0, 72.0] | 48.0 [24.0, 72.0] | 0.117 | |
| LAD, mm | 44.1±5.0 | 46.9±5.8 | <0.001 | 0.522 | 45.8±5.0 | 46.3±5.4 | 0.105 | |
| LVEF (%) | 56.0 [51.0, 60.0] | 57.0 [53.0, 61.0] | 0.036 | 0.281 | 57.3±6.3 | 56.8±5.8 | 0.097 | |
| Left atrial appendage thrombus | 8 (2.2) | 65 (69.9) | <0.001 | 1.989 | 2 (2.3) | 59 (67.8) | 1.888 | |
Data are presented as median [interquartile range], n (%), or mean ± standard deviation. TA, thoracoscopic ablation; CMP-IV, Cox-Maze IV procedure; PSM, propensity score matching; SMD, standardized mean difference; BMI, body mass index; NYHA, New York Heart Association; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age, and sex category; AF, atrial fibrillation; LAD, left atrium diameter; LVEF, left ventricular ejection fraction.
Matched population
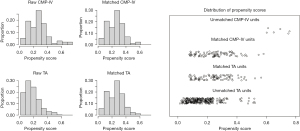
To minimize these potential biases, a total of 174 patients were eligible for 1:1 PSM from the TA and CMP-IV groups, respectively (Figure 1). The baseline characteristics of the matched patients are listed in Table 1. After propensity matching, the median age was 58.8±11.1 years in the TA group, higher than 58.4±8.9 years in CMP-IV group (P=0.793). Both groups were similar in gender, BMI, hypertension, diabetes, previous cerebrovascular accident, coronary artery disease, CHA2DS2-VASc score, previous catheter ablation, LA diameter, and LVEF.
Postoperative outcomes
Postoperative outcomes for the two groups are listed in Table 2. The length of intensive care unit (ICU) (20 vs. 30 hours, P<0.001) and hospital stay (6 vs. 10 days, P<0.001) was significantly shorter in the TA group. Complications, such as pneumonia, prolonged ventilation, acute renal failure, blood product transfusion, reoperation for bleeding and new pacemaker implantation, were higher in CMP-IV groups but not significantly different.
Table 2
| Postoperative event | All included patients | PSM patients | |||||
|---|---|---|---|---|---|---|---|
| TA (n=366) | CMP-IV (n=93) | P value | TA (n=87) | CMP-IV (n=87) | P value | ||
| Stroke | 1 | 0 | >0.999 | 0 | 0 | >0.999 | |
| Pneumonia | 4 | 5 | 0.025 | 2 | 4 | 0.681 | |
| Poor wound healing | 11 | 1 | 0.498 | 5 | 1 | 0.209 | |
| Prolonged ventilation | 5 | 4 | 0.160 | 1 | 3 | 0.620 | |
| Acute renal failure | 0 | 1 | 0.459 | 0 | 1 | >0.999 | |
| Blood product transfusion | 4 | 7 | 0.001 | 1 | 6 | 0.116 | |
| Reoperation for bleeding | 1 | 1 | 0.867 | 0 | 1 | >0.999 | |
| New pacemaker | 2 | 2 | 0.389 | 0 | 2 | >0.999 | |
| Mortality within 30 days | 0 | 0 | >0.999 | 0 | 0 | >0.999 | |
| ICU time, hour | 20.00 [18.00, 28.00] | 30.00 [24.00, 36.00] | <0.001 | 20.00 [18.00, 24.00] | 29.00 [24.00, 36.00] | <0.001 | |
| Hospital stays, day | 6.00 [5.00, 6.00] | 10.00 [8.00, 12.00] | <0.001 | 6.00 [5.00, 6.00] | 10.00 [8.00, 11.25] | <0.001 | |
Data are presented as number or median [interquartile range]. TA, thoracoscopic ablation; CMP-IV, Cox-Maze IV procedure; PSM, propensity score matching; ICU, intensive care unit.
Survival free from recurrence
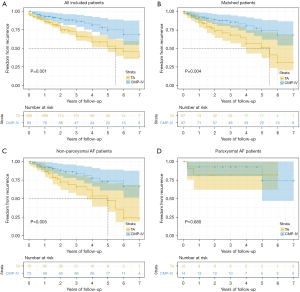
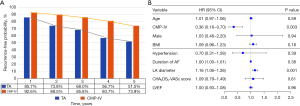
At discharge, continuous telemetric monitoring confirmed freedom from AF in 85 out of 87 patients in the CMP-IV group and 84 out of 87 patients in the TA group. The mean follow-up period was 31.5±22.1 months. At 1-year follow-up, 93.1% and 86.2% of patients maintained sinus rhythm in the CMP-IV and TA groups, respectively, while 88.5% and 74.7% of patients maintained sinus rhythm at 3-year follow-up. Kaplan-Meier estimation revealed that CMP-IV showed higher cumulative survival free from recurrence than TA both before and after matching (P=0.001, P=0.004) (Figure 2A,2B). With respect to the type of AF, there was no significant difference in freedom from recurrence of paroxysmal AF between the two groups (P=0.680), while CMP-IV demonstrated a higher freedom from recurrence than TA in non-paroxysmal AF patients (P=0.005) (Figure 2C,2D). Notably, there was no significant difference in freedom from recurrence between the two groups at only 1 year [0.926 (95% CI: 0.924–0.928) vs. 0.857 (95% CI: 0.853–0.860), log-rank test P=0.157] (Figure 3A).
Factors associated with postoperative recurrence
Univariable analysis of fourteen preoperative and perioperative variables was performed to determine potential factors contributing to first recurrence probability within the follow-up period. CMP-IV procedure, BMI, failure of catheter ablation, duration of AF and LA diameters were associated with recurrence (P<0.05). Variables deemed significant in univariate analysis and clinically relevant factors associated with AF containing age, gender, hypertension, and CHADS2-VASC scoring factors were included for further multivariable consideration. Using multivariable COX regression to adjust for clinically relevant covariates revealed that CMP-IV [hazard ratio (HR) 0.36, 95% CI: 0.18–0.70, P=0.003] was associated with decreased risk of recurrence, and increased left atrial size (HR 1.16, 95% CI: 1.06–1.26, P=0.001) was an independent predictor of postoperative recurrence (Figure 3B). In TA and CMP-IV treatment groups, increased left atrial size was associated with both increased risk of recurrence in TA (HR 1.13, 95% CI: 1.019–1.248, P=0.020) and CMP-IV groups (HR 1.39, 95% CI: 1.131–1.711, P=0.002).
Discussion
Transcatheter ablation is an effective and less invasive option for patients with AF. Unlike traditional surgical methods, TA eliminates the need for extracorporeal circulation or median sternotomy, thereby minimizing surgical trauma and enhancing patient acceptance. Despite numerous clinical studies showing TA’s superior success rates compared to catheter ablation (7,8), few studies have directly compared TA and CMP-IV. Our study demonstrates that TA has a faster recovery time and lower incidence of complications compared to CMP-IV. While the long-term effects of TA do not exhibit superiority, both treatments display comparable one-year success rates. In treating paroxysmal AF, CMP-IV and TA demonstrate similar efficacy, but CMP-IV has a higher success rate for non-paroxysmal AF patients. Furthermore, patients with a large left atrium face a relatively poor prognosis irrespective of the chosen treatment method.
The choice between bi-atrial ablation and left atrium box plus mitral isthmus ablation is a topic of debate in clinical practice (9,10). This study aimed to provide data for this discussion. The study’s TA group employed the left atrium box plus mitral valve isthmus ablation strategy via a three-circular and three-linear ablation route, while the CMP-IV group underwent standard bi-atrial ablation. The final follow-up results revealed better curative effects for bi-atrial ablation. We attribute this difference to four primary factors. First, TA is performed from the epicardium under the beating heart, with flowing blood dampening the ablation energy, leading to reduced transmurality (11). Second, in CMP-IV, ablation lines are completed entirely using ablation clamps, ensuring ablation quality. In contrast, in TA, some ablation lines can only be made using ablation pens, resulting in lower ablation quality than that of ablation clamps (12,13). Third, due to anatomical constraints, the mitral valve isthmus cannot be ablated from the epicardium, and only the Dallas line is feasible. However, the effect of the Dallas line remains controversial, and some studies show that adding the Dallas line does not increase the ablation success rate (14). Finally, left atrial ablation in TA compared to CMP-IV’s bi-atrial ablation differs in whether the right atrium is ablated or not. The role of the right atrium in AF remains disputed. While studies have identified some AF targets in the right atrium, particularly in long-duration patients, some indicate that it is only a bystander and does not lead directly to AF (15,16). Although our study’s data indicates that bi-atrial ablation has better curative effects than left atrial ablation, further research is needed to determine the role of the right atrium in AF.
Given the limitations of surgical ablation, which is not amenable to repeated procedures like catheter ablation, our routine clinical practice involves a CMP-IV procedure with bi-atrial ablation during open heart surgery. In the context of minimally invasive AF surgery, where anatomical constraints come into play, left atrial ablation combined with the Dallas lesion is undertaken. Nevertheless, we advocate for proactive exploration of appropriate methods for right atrial ablation in minimally invasive surgery (17). We caution against indiscriminate addition of ablation lines for right atrial ablation, as this has the potential to harm healthy atrial tissue. Such an approach may not necessarily enhance treatment success rates and could contribute to an increased incidence of postoperative atrial tachycardia or persistent AF (18).
The efficacy of TA as a stand-alone treatment for AF is currently inferior to that of the classic Cox-Maze procedure IV. However, this may be improved through several means, including enhancing the quality of ablation, refining the ablation route, and carefully selecting patients for treatment. Furthermore, there remains room for enhancing the minimally invasive nature of CMP-IV, ultimately improving the quality of life for patients with AF.
Acknowledgments
We would like to thank Mr Yuanzhe Ma, Saie Shen MD, and Ms Huihua Chen for their excellent experimental and clinical support.
Funding: The work was sponsored by
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bizhanov KA, Abzaliyev KB, Baimbetov AK, et al. Atrial fibrillation: Epidemiology, pathophysiology, and clinical complications (literature review). J Cardiovasc Electrophysiol 2023;34:153-65. [Crossref] [PubMed]
- Karamitanha F, Ahmadi F, Fallahabadi H. Difference Between Various Countries in Mortality and Incidence Rate of the Atrial Fibrillation Based on Human Development Index in Worldwide: Data From Global Burden of Disease 2010-2019. Curr Probl Cardiol 2023;48:101438. [Crossref] [PubMed]
- Wang R, Ning N, Wang S, et al. Real-world treatment patterns and stroke risks among patients with atrial fibrillation in China. Future Cardiol 2022;18:787-96. [Crossref] [PubMed]
- Churyla A, Passman R, McCarthy PM, et al. Staged hybrid totally thoracoscopic maze and catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:1961-5. [Crossref] [PubMed]
- Ma N, Lu R, Zhao D, et al. Left Atrial Appendage Fibrosis and 3-Year Clinical Outcomes in Atrial Fibrillation After Endoscopic Ablation: A Histologic Analysis. Ann Thorac Surg 2020;109:69-76. [Crossref] [PubMed]
- Mei J, Ma N, Ding F, et al. Complete thoracoscopic ablation of the left atrium via the left chest for treatment of lone atrial fibrillation. J Thorac Cardiovasc Surg 2014;147:242-6. [Crossref] [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [Crossref] [PubMed]
- Phan K, Phan S, Thiagalingam A, et al. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardiothorac Surg 2016;49:1044-51. [Crossref] [PubMed]
- Sef D, Trkulja V, Raja SG, et al. Comparing mid-term outcomes of Cox-Maze procedure and pulmonary vein isolation for atrial fibrillation after concomitant mitral valve surgery: A systematic review. J Card Surg 2022;37:3801-10. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329-41. [Crossref] [PubMed]
- Khoynezhad A, Warrier N, Worthington T, et al. A narrative review of hybrid ablation for persistent and longstanding persistent atrial fibrillation. Ann Transl Med 2021;9:947. [Crossref] [PubMed]
- Ma N, Ding S, Zeng L, et al. Immediate electrophysiological characteristics following modified thoracoscopic ablation via unilateral approach for non-valvular atrial fibrillation. Heart Vessels 2021;36:874-81. [Crossref] [PubMed]
- Wakasa S, Kubota S, Shingu Y, et al. Histological assessment of transmurality after repeated radiofrequency ablation of the left atrial wall. Gen Thorac Cardiovasc Surg 2014;62:428-33. [Crossref] [PubMed]
- Kim TH, Park J, Park JK, et al. Linear ablation in addition to circumferential pulmonary vein isolation (Dallas lesion set) does not improve clinical outcome in patients with paroxysmal atrial fibrillation: a prospective randomized study. Europace 2015;17:388-95. [Crossref] [PubMed]
- Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017;14:1087-96. [Crossref] [PubMed]
- Soni LK, Cedola SR, Cogan J, et al. Right atrial lesions do not improve the efficacy of a complete left atrial lesion set in the surgical treatment of atrial fibrillation, but they do increase procedural morbidity. J Thorac Cardiovasc Surg 2013;145:356-61; discussion 361-3. [Crossref] [PubMed]
- Zheng Z, Li H, Liu S, et al. Box lesion or bi-atrial lesion set for atrial fibrillation during thoracoscopic epicardial ablation. Interact Cardiovasc Thorac Surg 2022;34:1-8. [Crossref] [PubMed]
- Correia ETO, Barbetta LMDS, Mesquita ET. Extent of Left Atrial Ablation Lesions and Atrial Fibrillation Recurrence after Catheter Ablation - A Systematic Review and Meta-Analysis. Arq Bras Cardiol 2020;114:627-35. [Crossref] [PubMed]






