Comparison of the short-term outcomes between Y-incision aortic annular enlargement and traditional aortic annular enlargement techniques
Introduction
The term “patient-prosthesis mismatch” (PPM), although controversial, is recognized to describe a nonstructural dysfunction of the aortic valve prosthesis after aortic valve replacement (AVR) when a prosthetic aortic valve has an effective orifice area (EOA) that is significantly smaller than the area of the native aortic annulus, and is disproportionately small compared to the patient’s body surface area (BSA) (1,2). PPM has been associated with worse outcomes following both surgical and transcatheter AVR (TAVR), and therefore, the recent zeitgeist of surgeons has been slowly transitioning towards trying to implant larger prostheses (3-8). Prosthetic aortic valves, with mounted leaflets within a space-occupying housing, cause the EOA to be smaller than the native aortic annulus area, which has been a known factor in AVR since as early as the 1970s (2,9,10). The Nicks technique—the first proposed method of addressing this issue by enlarging the posterior aortic root—was introduced in 1970 to allow for a larger prosthesis by extending the aortotomy posteriorly in the noncoronary sinus through the annulus, and using patch closure (11,12). The Manouguian technique followed shortly thereafter in 1979, a similar technique which enlarges the posterior aortic root with an incision extending through the left-noncoronary commissure and into the anterior mitral leaflet, allowing for an aortic valve prosthesis up to two sizes larger than the native annulus could accommodate without enlargement (12,13). While these techniques are extra procedures which require increasing the cross-clamp and cardiopulmonary bypass times, studies over the past two decades have generally found that they can be performed without increasing mortality, especially in relatively healthy patients (14).
The Y-incision technique, which was first used in August 2020, introduced a new way to enlarge the aortic annulus by a larger degree than either the Nicks or Manouguian techniques, by up to 3–5 valve sizes (15-17). Although relatively new, the short-term outcomes of the Y-incision technique have shown it to be a safe and effective way to enlarge the aortic annulus in AVR surgery (9). In this study, we compared the outcomes of Y-incision aortic annular enlargement (AAE) to traditional AAE in AVR, with operative mortality and efficacy of annular enlargement as the primary outcomes. We hypothesized that the Y-incision technique is more effective in enlarging the aortic annulus than traditional techniques.
Methods
Patients
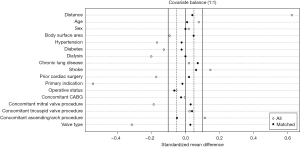
Between February 2011 and June 2022, 380 patients underwent AVR with AAE using either the Y-incision technique for AAE (Y-incision group, n=110), or traditional techniques (Traditional group, n=270), including Nicks [63% (171/270)], Manouguian [34% (91/270)], and others [3% (8/270)]. Propensity score matching was then performed, controlling for age, sex, BSA, hypertension, diabetes, dialysis, chronic lung disease, stroke, prior cardiac surgery, primary indication, operative status, concomitant procedures, and prosthesis type, to generate a balanced cohort made up of 103 pairs (n=206). Standardized mean differences of less than 0.1 indicate acceptable balance of the covariates between the matched groups (Figure 1). Patients with any concomitant procedures were included in the match, but patients with endocarditis as the primary indication were excluded due to the anatomical challenges abscess formation can present on the surgical technique of AAE.
Interventions
The Nicks and Manouguian techniques for AAE were used for the entire study period, while Y-incision was only used from its introduction in August 2020 and onwards. The decision to perform AAE was determined by the surgeon’s judgment in consideration of multiple factors, including the size of the patient’s native aortic annulus (measured using prosthesis manufacturer sizers intraoperatively), the patient’s BSA, and the patient’s expected postoperative activity level. Among the traditional techniques for AAE, the decision to use Nicks or Manouguian technique came down to surgeon preference. After August 2020, surgeons adopted the Y-incision technique in place of the traditional techniques to varying degrees. Y-incision tended to be used preferentially to enable a greater degree of annular enlargement, and its usage increased over time. By the last year of the study in 2022, 82% (47/57) of AAEs were performed using the Y-incision technique.
Study design
This study was a retrospective cohort study comparing the short-term outcomes of AVR with AAE using either traditional techniques or the Y-incision technique of AAE for the study period of February 2011 to June 2022. Data was obtained through the Society of Thoracic Surgery Data Warehouse to identify the relevant cohort and determine the perioperative, operative, and postoperative variables, and was supplemented with medical record review. Primary outcomes included perioperative complications/outcomes and short-term survival. The secondary outcomes were hemodynamic performance including mean aortic valve gradients, aortic valve areas, and incidence of PPM on one-year follow-up echocardiography.
PPM was defined by the current standard definitions as follows: non-obese patients [body mass index (BMI) <30 kg/m2] were classified as having severe patient prosthesis mismatch if their indexed EOA (iEOA) was less than 0.65 cm2/m2, and moderate if their iEOA was less than 0.85 cm2/m2, while obese patients (BMI >30 kg/m2) were classified as having severe patient prosthesis mismatch if their iEOA was less than 0.55 cm2/m2, and moderate if their iEOA was less than 0.70 cm2/m2. The Institutional Review Board or equivalent ethics committee of the University of Michigan, Michigan Medicine (Ann Arbor, MI, USA) (HUM00211344, 1/21/2022) approved the study protocol and publication of data. Patient written consent for the publication of the study data was waived by the IRB due to minimal risk to patients.
Data collection
Data was obtained through the Society of Thoracic Surgery Data Warehouse to identify the relevant cohort and determine the perioperative, operative, and postoperative variables. This data was supplemented with medical chart review. Echocardiography at one-year post-operation was performed and recorded as a part of routine follow-up. Survival and reoperation data were collected by medical record review, and additional survival data was supplied by the Michigan Death Index data through December 12th, 2022, and the National Death Index data through to December 31st, 2018 (18). All derived data collected which support the findings of this study are available by request from the corresponding author.
Statistical analysis
Continuous data was presented as median [interquartile range (IQR), 25%, 75%] and categorical data as n (%). Univariable comparisons between groups were performed using chi-square tests for categorical data. Kruskal-Wallis one-way analysis of variance tests were performed for continuous data. Kaplan-Meier curves were generated to estimate survival. P values less than 0.05 were considered statistically significant. All statistical calculations used SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Preoperative and demographic data
Preoperative comorbidities and demographic characteristics of the propensity score matched groups are described in Table 1. The median age of the matched cohort was 64 years and 36% (74/206) of patients were male. The Traditional and Y-incision groups were similar across all measured preoperative or demographic characteristics including age, sex, BSA, prior cardiac surgery, and other comorbidities. Society of Thoracic Surgeons Preoperative Risk of Mortality scores were similar between groups.
Table 1
| Variables | Traditional (n=103) | Y-incision (n=103) | P value |
|---|---|---|---|
| Age, years | 65 [58, 71] | 63 [57, 70] | 0.47 |
| Sex (male) | 37 [36] | 37 [36] | >0.99 |
| Body mass index | 30 [26, 36] | 29 [25, 34] | 0.33 |
| Body surface area | 2.0 [1.8, 2.2] | 2.0 [1.8, 2.2] | 0.91 |
| Hypertension | 74 [72] | 73 [71] | 0.88 |
| Chronic lung disease | 18 [17] | 21 [20] | 0.59 |
| Last creatinine levels | 0.8 [0.8, 1.0] | 0.9 [0.8, 1.1] | 0.21 |
| Dialysis | 0 | 0 | >0.99 |
| Diabetes | 31 [30] | 30 [29] | 0.88 |
| Prior stroke | 9 [8.7] | 11 [11] | 0.64 |
| Prior cardiac surgery | 31 [30] | 32 [31] | 0.88 |
| Prior aortic valve replacement | 18 [17] | 19 [18] | 0.86 |
| Case status | 0.82 | ||
| Elective | 92 [89] | 93 [90] | |
| Urgent | 11 [11] | 10 [9.7] | |
| Primary indication | 0.76 | ||
| Aortic stenosis | 97 [94] | 98 [95] | |
| Aortic insufficiency | 6 [5.8] | 5 [4.9] | |
| STS-PROM score (%) | 1.4 [0.8, 2.5] | 1.5 [0.9, 2.4] | 0.98 |
Data presented as median [interquartile range] for continuous variables and number [percentage] for categorical variables. STS-PROM, Society of Thoracic Surgeons Preoperative Risk of Mortality.
Intraoperative data
The median native aortic annulus diameter in both the Traditional and Y-incision groups, measured intraoperatively with prosthesis manufacturer sizers, was 21 mm. The median degree of prosthesis sizes upsized was one in the Traditional group and three in the Y-incision group, making the median prosthesis size 23 in the Traditional group and 27 in the Y-incision group. There was a similar distribution of the usage of bioprosthetic and mechanical valves between the groups. Full distribution of valve models and sizes across the two groups are described in Table S1. Other intraoperative factors including cardiopulmonary bypass times, cross clamp times, rates of concomitant procedures, and usage of most blood products were similar between the two groups, but more patients in the Y-incision group received cryoprecipitate (Table 2).
Table 2
| Variables | Traditional (n=103) | Y-incision (n=103) | P value |
|---|---|---|---|
| Cardiopulmonary bypass time | 162 [136, 210] | 177 [148, 223] | 0.071 |
| Cross clamp time | 136 [108, 181] | 149 [120, 183] | 0.13 |
| Annular enlargement technique | |||
| Nicks | 65 [63] | 0 | |
| Manouguian | 34 [33] | 0 | |
| Other | 4 [3.9] | 0 | |
| Y-incision | 0 | 103 [100] | |
| Native aortic annulus size (mm) | 21 [21, 23] | 21 [21, 23] | 0.69 |
| Number of sizes upsized | 1 [1, 1] | 3 [2, 3] | <0.001 |
| Implanted prosthesis size | 23 [23, 25] | 27 [25, 29] | <0.001 |
| Prosthesis type | 0.83 | ||
| Biological | 91 [88] | 90 [87] | |
| Prosthesis size | 23 [23, 25] | 27 [25, 29] | <0.001 |
| Size 19 | 1 [1.0] | 0 | |
| Size 21 | 12 [12] | 0 | |
| Size 23 | 39 [38] | 7 [6.8] | |
| Size 25 | 31 [30] | 20 [19] | |
| Size 27 | 6 [5.8] | 33 [32] | |
| Size 29 | 0 | 30 [29] | |
| Mechanical | 12 [12] | 13 [13] | |
| Prosthesis size | 23 [21, 24] | 27 [27, 27] | <0.001 |
| Size 19 | 1 [1.0] | 0 | |
| Size 21 | 4 [3.9] | 0 | |
| Size 23 | 4 [3.9] | 2 [1.9] | |
| Size 25 | 2 [1.9] | 1 [1.0] | |
| Size 27 | 1 [1.0] | 9 [8.7] | |
| Size 29 | 0 | 1 [1.0] | |
| Concomitant procedures | |||
| Coronary artery bypass grafting | 21 [20] | 20 [19] | 0.86 |
| Ascending/arch procedure | 22 [21] | 20 [19] | 0.73 |
| Mitral valve procedure | 10 [9.7] | 11 [11] | 0.82 |
| Tricuspid valve procedure | 6 [5.8] | 7 [6.8] | 0.77 |
| Blood products used | 34 [33] | 32 [31] | 0.77 |
| Red blood cells | 22 [21] | 23 [22] | 0.87 |
| Platelets | 22 [21] | 25 [24] | 0.62 |
| Fresh frozen plasma | 14 [14] | 19 [18] | 0.34 |
| Cryoprecipitate | 3 [2.9] | 10 [9.7] | 0.045 |
Data presented as median [interquartile range] for continuous variables and number [percentage] for categorical variables.
Perioperative data
All measured perioperative complications including reoperation for bleeding, stroke, atrial fibrillation, complete heart block requiring pacemaker implantation, pneumonia, sepsis, and renal failure requiring dialysis were similar between the two groups, as was the time spent on mechanical ventilation, length of intensive care unit stay, and length of hospital stay. Operative mortality in the entire cohort was 3.9% (8/206) and was not different between the Traditional [4.9% (5/103)] and Y-incision [2.9% (3/103)] groups (P=0.72).
One-year follow-up
Kaplan-Meier survival was similar between the groups, with an estimated one-year survival of 95% [95% confidence interval (CI): 89%, 98%] in the Traditional group compared to 97% (95% CI: 90%, 99%) in the Y-incision group (Figure 2, P=0.54). There was no reoperation for valve degeneration at 12 months in either of the groups. Causes of death for the patients who died during the follow-up period (n=10) are described in Table S2.

Echocardiographic data
The Traditional group and Y-incision group had comparable mean aortic valve gradients and aortic valve areas as measured on preoperative echocardiography. The Y-incision group had significantly lower mean aortic valve gradients intraoperatively immediately post-procedure [6 mmHg (IQR: 5, 7 mmHg) vs. 7 mmHg (IQR: 6, 10 mmHg)] and on one-year follow-up [7 mmHg (IQR: 5, 9 mmHg) vs. 10 mmHg (IQR: 8, 11 mmHg)] than the Traditional group. The aortic valve area was significantly larger in the Y-incision group on one-year follow-up at 2.2 cm2 (IQR: 1.9, 2.4 cm2) compared to 1.8 cm2 (IQR: 1.6, 2.1 cm2) in the Traditional group (P=0.007). The iEOA was greater in the Y incision group at 1.05 cm2/m2 (IQR: 0.92, 1.33 cm2/m2) compared to 0.86 cm2/m2 (IQR: 0.78, 1.03 cm2/m2) in the Traditional group (P=0.002). There was a higher incidence of moderate or severe patient prosthesis mismatch in the Traditional group at 23% (5/22) compared to 5.5% (3/55) in the Y-incision group, which had no severe PPM (P=0.039). The incidence of mitral regurgitation post-operatively was similar between the groups and was improved compared to preoperative mitral regurgitation in both groups (Table 3).
Table 3
| Variables | Traditional | Y-incision | P value |
|---|---|---|---|
| Preoperative | n=94 | n=91 | |
| Ejection fraction (%) | 65 [59, 70] | 60 [58, 65] | 0.035 |
| AV mean gradient (mmHg) | 38 [28, 51] | 36 [25, 46] | 0.31 |
| Aortic valve area (cm2) | 0.8 [0.6, 1.0] | 0.9 [0.7, 1.1] | 0.15 |
| Mitral regurgitation | 0.51 | ||
| None | 31 [33] | 32 [35] | |
| Trace | 26 [28] | 27 [30] | |
| Mild | 29 [31] | 19 [21] | |
| Moderate | 6 [6.4] | 10 [11] | |
| Severe | 2 [2.1] | 3 [3.3] | |
| Intraoperative post-procedure | n=87 | n=90 | |
| AV mean gradient (mmHg) | 7 [6, 10] | 6 [5, 7] | <0.001 |
| One-year postoperative | n=44 | n=61 | |
| Ejection fraction (%) | 60 [60, 65] | 60 [55, 65] | 0.63 |
| AV mean gradient (mmHg) | 10 [8, 11] | 7 [5, 9] | <0.001 |
| Mitral regurgitation | 0.65 | ||
| None | 10 [23] | 20 [33] | |
| Trace | 19 [43] | 25 [41] | |
| Mild | 13 [30] | 13 [21] | |
| Moderate | 2 [4.5] | 3 [4.9] | |
| Severe | 0 | 0 | |
| Hemodynamics | n=22 | n=55 | |
| Aortic valve area (cm2) | 1.8 [1.6, 2.1] | 2.2 [1.9, 2.4] | 0.007 |
| Indexed effective orifice area (cm2/m2) | 0.86 [0.78, 1.03] | 1.05 [0.92, 1.33] | 0.002 |
| Moderate or severe PPM | 5 [23] | 3 [5.5] | 0.039 |
| Moderate PPM | 4 [18] | 3 [5.5] | |
| Severe PPM | 1 [4.5] | 0 |
Data presented as median [interquartile range] for continuous variables and number [percentage] for categorical variables. AV, aortic valve; PPM, patient-prosthesis mismatch.
Discussion
In this study, we found that the short-term outcomes of AVR with AAE are largely comparable whether the traditionally-used surgical techniques for AAE (Nicks and Manouguian) were used compared to the more newly-introduced Y-incision technique. There was no significant difference in operative mortality between the groups, and no differences in perioperative complications, but the Y-incision technique was more effective in upsizing the prosthetic valve (upsizing three valve sizes vs. one valve size) (Tables 2,4). Short-term survival was also comparable between the groups (Figure 2). Echocardiographic data showed comparable preoperative mean aortic valve gradients, but significantly lower gradients in the Y-incision group, both intraoperatively, post-procedure, and on one-year follow-up echocardiogram. The Y-incision group also had a significantly larger aortic valve area on one-year follow-up echocardiogram with a greater iEOA and less incidence of moderate/severe PPM. On one-year follow-up, mitral regurgitation was improved compared to preoperative condition in both groups, and the incidence of mitral regurgitation was similar whether the Y-incision or Traditional annular enlargement techniques were used (Table 3).
Table 4
| Variables | Traditional (n=103) | Y-incision (n=103) | P value |
|---|---|---|---|
| Reoperation due to bleeding | 1 [1.0] | 5 [4.9] | 0.22 |
| Stroke | 3 [2.9] | 2 [1.9] | >0.99 |
| Atrial fibrillation | 34 [33] | 39 [38] | 0.47 |
| Pacemaker implantation | 3 [2.9] | 0 | 0.25 |
| Prolonged ventilation | 11 [11] | 13 [13] | 0.66 |
| Pneumonia | 4 [3.9] | 6 [5.8] | 0.75 |
| Sepsis | 4 [3.9] | 2 [1.9] | 0.68 |
| Renal failure on dialysis | 5 [4.9] | 5 [4.9] | >0.99 |
| Length of hospital stay (days) | 7 [5, 11] | 9 [6, 11] | 0.052 |
| Intensive care unit stay (hours) | 65 [41, 117] | 73 [37, 122] | 0.43 |
| Ventilator time (hours) | 5 [4, 10] | 4 [3, 12] | 0.38 |
| Operative mortality | 5 [4.9] | 3 [2.9] | 0.72 |
Data above is presented as median [25%, 75%] for continuous data and n [%] for categorical data.
Our study is the first to directly compare the short-term outcomes of AAE between the established operative techniques, Nicks and Manouguian, and the newly-introduced Y-incision technique. The Nicks and Manouguian techniques have seen widespread utilization since they were developed in the 1970s, and there have been numerous studies which evaluated their safety profiles in comparison with AVR without annular enlargement (19-24). By contrast, the Y-incision technique has only been used since August 2020, and has not yet had widespread use. We recently described our experience using the Y-incision technique in the first fifty patients undergoing the procedure by a single surgeon, and found excellent short-term outcomes with no operative mortality, minimal complications, and excellent hemodynamics (9). This study analyzed the generalization of Y-incision AAE at the University of Michigan Hospital by including Y-incision AAE cases performed by all surgeons. With data of all surgeons doing Y-incision AAE, we found that the biggest difference between the traditional AAE techniques and Y-incision AAE is the degree of valve sizes able to be upsized. In our study, the median number of valve sizes upsized with the traditional techniques was only one, but with the Y-incision technique, patients on average had a prosthesis that was three sizes larger than their native annulus could hold, and the Y-incision technique has been used in the past to enlarge the aortic annulus up to five valve sizes (17).
Based on this, we arrive at the core question that our study aims to answer: can more aggressive AAE be regularly performed to use the largest valve size possible, without compromising short-term outcomes? The rates of perioperative complications were similar between the groups and the operative mortality in the Y-incision group was close to half of that in the Traditional group (operative mortality: 2.9% vs. 4.9%), which was overall similar to what has been described with the Nicks and Manouguian techniques in the literature (25). One concern of AAE in general is the possible disruption of the conducting fibers in the interventricular septum, causing heart block. Our study shows that when performed correctly, Y-incision AAE can be used to enlarge the aortic annulus by a great degree with minimal risk (0%) of interrupting the conducting system, consistent with previous findings (9). This was likely due to the posterior enlargement of the aortic annulus not disturbing the conducting system, which is located anteriorly. Mitral regurgitation is also sometimes a concern with AAE due to interruption of the anterior mitral leaflet (26). With our study, we found comparable incidence of mitral regurgitation between the Traditional and Y-incision groups both preoperatively and on one-year follow-up. Post-operatively, there was decreased moderate and severe mitral regurgitation in both groups, indicating that despite the Y-incision technique enlarging the aortic annulus more aggressively, there was no increased risk of mitral regurgitation. In the Y-incision group, there was no acute myocardial infarction or acute coronary issues postoperatively. We have not seen any left coronary torsion with Y-incision annular enlargement thus far.
By showing comparable short-term outcomes between the Traditional and Y-incision groups, and hemodynamic advantages of the Y-incision technique due to being able to implant larger prostheses, confirmed with echocardiography, we provide evidence that the Y-incision technique can be routinely used in place of the traditional techniques of AAE. Our group has previously demonstrated both short- and long-term survival benefits of large prostheses compared to smaller ones in a large study of patients undergoing AVR (27). The aortic annulus area is decreased relative to the native aortic annulus area whenever a prosthetic valve is implanted, due to the bulky struts and sewing ring, a fact which has been described since the 1970s (2). We have previously described how the degree of this decrement is much more significant in smaller valves, as the aortic annulus area is decreased by around half whenever a size 23 prosthesis or smaller is implanted without enlargement, and by a third when a size 25 valve is implanted (9). This study showed that when the Nicks or Manouguian techniques are used, the median prosthesis implanted is still a size 23, showing that even when these AAE techniques were performed, the aortic annulus area was still being decreased significantly. With a median prosthesis size of 27 in the Y-incision group, it is unsurprising that we observed superior hemodynamics in the Y-incision group compared to the Traditional group, with significantly lower mean aortic valve gradients, larger aortic valve areas, greater iEOA, and less moderate/severe PPM on one-year follow-up echocardiography (Table 3). Based on our results, the University of Michigan Hospital now utilizes the Y-incision technique as our first choice for AAE.
Despite the large degree of annular enlargement which is possible with the Y-incision technique, there were still three patients in our study that underwent Y-incision AAE who had moderate PPM on follow-up echocardiogram. One patient had a size 29 Magna Ease valve (Edwards Lifesciences, Irvine, CA, USA) implanted, but even this was not able to offset his large BMI and BSA of 47.5 kg/m2 and 2.96 m2, respectively, to prevent moderate PPM. The second patient had a size 27 Top Hat valve (Sulzer, Carbomedics, Austin, TX, USA), which is the largest size, but his echocardiogram, which was performed at an outside hospital, was recorded at only 1.31 cm2, which is an unexpected measurement. The last was a patient with a 21 mm aortic annulus who had a size 25 Avalus valve (Medtronic, Minneapolis, MN, USA) implanted with an iEOA of 0.81 cm2/m2 with a left ventricular ejection fraction of 55–60%. It is possible that this patient could have benefitted from more aggressive enlargement to have a size 27 valve placed instead.
There are still two significant questions yet to be answered regarding this comparison between the Y-incision technique and traditional AAE techniques. The first is pertaining to how these hemodynamic advantages convert to long-term outcomes. Hemodynamic advantages do not always correspond with a long-term survival benefit, so further studies will need to be conducted as more patients who have undergone Y-incision AAE reach longer follow-up. The second consideration is the impact of AAE technique on hemodynamics following future valve-in-valve (V-in-V) TAVR. As studies find that low BSA-indexed aortic valve areas after TAVR are associated with worse outcomes, the conversation of how to best prepare patients for future interventions becomes more important (8). While we speculate that being able to implant larger prostheses with the Y-incision technique for annular enlargement would lead to downstream improvement of hemodynamics following any subsequent interventions, future studies looking at AAE patients who have undergone V-in-V TAVR will be needed to ascertain if there are advantages associated with having a larger prosthesis after the initial surgical AVR.
Our study was limited as a retrospective cohort study—the decision to perform traditional aortic annular techniques versus the Y-incision technique was based on surgeon preference and not random, and the Y-incision technique was not used for the first nine years of the study period. Early survival was assessed by using the Michigan Death Index and National Death Index databases combined with manual chart review, but this method may not have documented 100% of deaths. We did not have echocardiographic data available for all patients due to loss to follow-up, echocardiograms being performed at other institutions, or poor image quality. Out of the patients for whom we did have echocardiographic data available, aortic valve area was often not able to be measured. We did not have data for late readmissions or New York Heart Association heart failure classification on follow-up visits, so our follow-up data was limited only to survival, reoperation, and echocardiographic data.
Conclusions
The Y-incision technique was as safe as the traditional techniques for AAE and was more effective in enlarging the aortic annulus. The Y-incision technique could be preferentially used in place of the traditional techniques of AAE for patients with a normal aortic annulus.
Acknowledgments
Funding: B.Y. is funded by
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 2006;92:1022-9. [Crossref] [PubMed]
- Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation 1978;58:20-4. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41. [Crossref] [PubMed]
- Rao V, Jamieson WR, Ivanov J, et al. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation 2000;102:III5-9. [Crossref] [PubMed]
- Bilkhu R, Jahangiri M, Otto CM. Patient-prosthesis mismatch following aortic valve replacement. Heart 2019;105:s28-33. [Crossref] [PubMed]
- Fallon JM, DeSimone JP, Brennan JM, et al. The Incidence and Consequence of Prosthesis-Patient Mismatch After Surgical Aortic Valve Replacement. Ann Thorac Surg 2018;106:14-22. [Crossref] [PubMed]
- Walther T, Rastan A, Falk V, et al. Patient prosthesis mismatch affects short- and long-term outcomes after aortic valve replacement. Eur J Cardiothorac Surg 2006;30:15-9. [Crossref] [PubMed]
- Herrmann HC, Daneshvar SA, Fonarow GC, et al. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J Am Coll Cardiol 2018;72:2701-11. [Crossref] [PubMed]
- Yang B, Ghita C, Makkinejad A, et al. Early outcomes of the Y-incision technique to enlarge the aortic annulus 3 to 4 valve sizes. J Thorac Cardiovasc Surg 2022; [Crossref]
- Ghanta RK, Kron IL. Patient-prosthesis mismatch: surgical aortic valve replacement versus transcatheter aortic valve replacement in high risk patients with aortic stenosis. J Thorac Dis 2016;8:E1441-3. [Crossref] [PubMed]
- Nicks R, Cartmill T, Bernstein L. Hypoplasia of the aortic root. Thorax 1970;25:339-46. [Crossref] [PubMed]
- Grubb KJ. Aortic root enlargement during aortic valve replacement: nicks and manouguian techniques. Oper Tech Thorac Cardiovasc Surg 2015;20:206-18.
- Manouguian S, Seybold-Epting W. Patch enlargement of the aortic valve ring by extending the aortic incision into the anterior mitral leaflet. New operative technique. J Thorac Cardiovasc Surg 1979;78:402-12.
- Lazar HL. Aortic Root Enlargement-Is It a Safe and Effective Approach to Prevent Patient-Prosthesis Mismatch and Is It for Everyone? Can J Cardiol 2019;35:707-9. [Crossref] [PubMed]
- Yang B. A novel simple technique to enlarge the aortic annulus by two valve sizes. JTCVS Tech 2021;5:13-6. [Crossref] [PubMed]
- Yang B, Naeem A A. Y Incision and Rectangular Patch to Enlarge the Aortic Annulus by Three Valve Sizes. Ann Thorac Surg 2021;112:e139-41. [Crossref] [PubMed]
- Yang B, Ghita C, Palmer S. Y-incision Aortic Root Enlargement With Modified Aortotomy Upsizing the Annulus by 5 Valve Sizes. Ann Thorac Surg 2022;114:e479-81. [Crossref] [PubMed]
- Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index. Available online: https://www.cdc.gov/nchs/ndi/index.htm
- Sommers KE, David TE. Aortic valve replacement with patch enlargement of the aortic annulus. Ann Thorac Surg 1997;63:1608-12. [Crossref] [PubMed]
- Kulik A, Al-Saigh M, Chan V, et al. Enlargement of the small aortic root during aortic valve replacement: is there a benefit? Ann Thorac Surg 2008;85:94-100. [Crossref] [PubMed]
- Okamoto Y, Yamamoto K, Sugimoto T, et al. Early and Late Outcomes of Aortic Valve Replacement with Aortic Annular Enlargement: A Propensity Analysis. Thorac Cardiovasc Surg 2016;64:410-7. [Crossref] [PubMed]
- Rocha RV, Manlhiot C, Feindel CM, et al. Surgical Enlargement of the Aortic Root Does Not Increase the Operative Risk of Aortic Valve Replacement. Circulation 2018;137:1585-94. [Crossref] [PubMed]
- Mehaffey JH, Hawkins RB, Wegermann ZK, et al. Aortic Annular Enlargement in the Elderly: Short and Long-Term Outcomes in the United States. Ann Thorac Surg 2021;112:1160-6. [Crossref] [PubMed]
- Shih E, DiMaio JM, Squiers JJ, et al. Outcomes of aortic root enlargement during isolated aortic valve replacement. J Card Surg 2022;37:2389-94. [Crossref] [PubMed]
- Yu W, Tam DY, Rocha RV, et al. Aortic Root Enlargement Is Safe and Reduces the Incidence of Patient-Prosthesis Mismatch: A Meta-analysis of Early and Late Outcomes. Can J Cardiol 2019;35:782-90. [Crossref] [PubMed]
- Imanaka K, Takamoto S, Furuse A. Mitral regurgitation late after Manouguian's anulus enlargement and aortic valve replacement. J Thorac Cardiovasc Surg 1998;115:727-9. [Crossref] [PubMed]
- Yang B, Makkinejad A, Fukuhara S, et al. Stentless Versus Stented Aortic Valve Replacement for Aortic Stenosis. Ann Thorac Surg 2022;114:728-34. [Crossref] [PubMed]






