Sex differences in long-term outcomes following surgery for acute type A aortic dissection: a systematic review and meta-analysis
Introduction
Acute type A aortic dissection (ATAAD) is a life-threatening condition with a 50% mortality rate before reaching a specialist center (1), where emergency life-saving surgery then carries an additional 9–26% mortality rate (2-6). Important sex-related differences in presentation and management of ATAAD have been described (7,8). Whether this then translated into in-hospital outcome differences was unclear as there was conflicting data from international registries, multi-center, and single-center studies (9-13). Recent updated analyses from the Canadian Thoracic Aortic Collaborative (CTAC) have demonstrated that while historically women had worse outcomes than men following aortic arch surgery, including surgery for ATAAD, this outcome gap has decreased and been eliminated over time (14). Furthermore, updated data from the International Registry for Aortic Dissections (IRAD) also showed reduction of sex differences in operative mortality amongst surgically treated ATAAD patients (9). In fact, recent systematic reviews and meta-analyses corroborate that early outcomes between the sexes have now become comparable (15,16).
Questions remain as to whether long-term outcomes differ between men and women following surgery for ATAAD. Sex-stratified analyses of patients receiving surgery for ATAAD have shown conflicting evidence (9-11,17), with some showing female sex as an independent predictor of worse long-term mortality (10), while other reports show that, among hospital survivors, women have better long-term outcomes than men (18,19). Aggregation of these studies through meta-analysis may provide clarity.
The first meta-analysis of long-term sex differences in patients undergoing surgery for ATAAD reported comparable overall survival between the sexes and higher rates of reoperation in male ATAAD patients (15). However, this analysis included a limited number of studies (five for long-term mortality and two studies for long-term reoperation). Another meta-analysis reported worse long-term survival in women, but several studies were not included, and long-term reoperation was not assessed (20). Therefore, sex differences in long-term outcomes after ATAAD remain controversial. In this systematic review and meta-analysis, we aim to definitively aggregate updated evidence on sex differences in long-term mortality and reoperation.
Methods
Literature search strategy
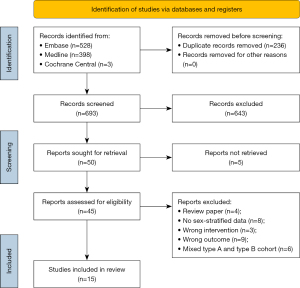
A systematic review and meta-analysis were conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) (21). A systematic review was initiated by searching Medline, Embase, and Cochrane Central from January 1, 2000, to March 15, 2023. Articles prior to 2000 were excluded due to changing cardiac surgery practice including the widespread combined use of contemporary perfusion techniques and systemic hypothermic circulatory arrest which have improved safety. A professional research librarian from our institution was consulted to review and optimize search terms. Both Medical Subject Heading (MeSH) and title, abstract, subject heading, and keyword were used to create search terms. A complete list of search terms for each database is provided in Appendix 1, Table S1.
Eligibility criteria
Relevant studies that met the inclusion criteria were those that included patients who underwent aortic surgery for the diagnosis of ATAAD, performed long-term follow-up of mortality or reoperation outcomes, and reported outcomes stratified by sex. Exclusion criteria included studies with data limited to a specific patient population (e.g., young patients, older patients, Marfan’s patients), acute aortic dissection without differentiation of type A vs. type B, chronic dissection, no stratification of outcomes by sex, and no long-term follow-up. Moreover, review articles without original data and conference abstracts were excluded. Title and abstract screening of articles from the systematic literature search was performed independently by two authors (Bhatt N and Rocha RV) in Covidence (Melbourne, Australia). Full-text review was also independently performed by Bhatt N and Rocha RV. Discrepancies in assessment were resolved by discussion and consensus.
Data extraction and critical appraisal
Data was extracted independently from text, tables, and figures. The primary data variables of interest were long-term mortality and reoperation. For studies with published Kaplan-Meier curves comparing outcomes between the sexes, all graphs were saved digitally and the data from the number at risk table was extracted. From published Kaplan-Meier curves, we used a validated algorithm to reconstruct individual patient-level survival data (22). Additionally, preoperative baseline characteristics and intraoperative variables were extracted from included studies. Continuous variables that were reported as median and interquartile range (IQR) were converted to mean ± standard deviation (SD) using a validated estimation method (23). A full list of extracted data variables is provided in Appendix 2, Table S2.
The validated Newcastle-Ottawa Scale (NOS) was used for quality and risk of bias assessment for nonrandomized cohort studies (24). NOS scores graded participant selection (maximum four stars), comparability between studied groups (maximum two stars), and assessment of outcome and follow-up (maximum three stars), for a maximum score of nine stars.
Statistical analysis
Continuous variables were reported as mean ± SD and categorical variables were reported as frequency (percentages). Extracted preoperative and intraoperative variables were aggregated using random effects models in Review Manager (RevMan) (version 5.4). Sex differences were assessed using pooled risk ratio (RR) (categorical variables) or mean difference (continuous variables), with men being the reference group. Heterogeneity across studies was assessed by calculating I2, with values of <25%, 25–75%, and >75% being interpreted as low, moderate, and high heterogeneity, respectively. Publication bias for preoperative characteristics, intraoperative variables, and early post-operative outcomes was assessed for analyses where at least 10 studies were included by plotting funnel plots in RevMan and performing Egger’s test in R statistical software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) using the ‘metafor’ package (version 4.2.0).
Sex differences in long-term outcomes were analyzed by reconstructing individual patient-level data for survival and reoperation outcomes, which was then aggregated to generate pooled Kaplan-Meier curves and obtain overall estimates of sex-specific survival and freedom from reoperation. We also performed a landmark analysis starting at 1-year following surgery, to exclude the influence of perioperative outcomes and the early patient course, and to assess sex differences in long-term outcomes in patients who were alive at 1-year.
Life expectancy for males and females in the general Canadian population were retrieved from data published by Statistics Canada (25). This data represented life expectancy for all male and female Canadians at a specific age. After adjusting for the average age of male and female patients receiving surgery for ATAAD, yearly life expectancies were plotted against the pooled Kaplan-Meier curve for both sexes.
Two separate sensitivity analyses were performed by excluding studies that performed propensity score matching, and including only studies rated as moderate to good quality according to our NOS assessment (overall score ≥6). All analysis of long-term outcomes was performed using R statistical software and Prism 9 (version 9.4.1; GraphPad, Boston, MA, USA). Significance level was taken as P<0.05.
Results
Quantity and quality of evidence
Our initial systematic search produced 929 studies of which 236 were duplicates and excluded prior to screening. Title and abstract screening resulted in 50 articles which were sought for full text review. The full text for five articles could not be retrieved via institutional library access. The remaining 45 articles underwent full-text review and 15 articles met inclusion criteria and were included in the qualitative synthesis and quantitative analysis. Table 1 outlines the characteristics of included studies. Across the 15 included studies, there were 7,608 male and 3,989 female patients. Of these, 12 reported single-center retrospective data (10,11,19,26-34), one reported multi-center data 35, and two reported on large multi-center national or international datasets (9,17). A total of 13 studies published Kaplan-Meier curves for survival (9-11,19,26-28,30-35) and four studies published curves for reoperation (9,17,19,28) and were subsequently included in the respective pooled Kaplan-Meier analyses. Two studies included cohorts starting from the earliest timepoint and followed patients over the longest time span from 1996–2018 (9,32).
Table 1
| Studies | Type | Country/region | Matched | Time | Male, n | Female, n |
|---|---|---|---|---|---|---|
| Gasser et al. | Single-center | Austria | No | 2000–2020 | 268 | 126 |
| Huckaby et al. | Multi-center, IRAD | Multi-national | No | 1996–2018 | 1,854 | 969 |
| Liu et al. | Single-center | China | No | 2002–2016 | 167 | 68 |
| Li et al. | Single-center | China | Yes | 2009–2014 | 451 | 302 |
| Norton et al. | Single-center | United States | No | 1996–2018 | 444 | 206 |
| Rios et al. | Single-center | Brazil | No | 2006–2016 | 63 | 24 |
| Sabashnikov et al. | Single-center | United States | Yes | 2006–2015 | 153 | 87 |
| Suzuki et al. | Single-center | Japan | No | 2004–2016 | 156 | 147 |
| Yousef et al. | Single-center | United States | No | 2007–2021 | 361 | 240 |
| Conway et al. | Multi-center, STS database | United States | No | 2000–2010 | 172 | 79 |
| Friedrich et al. | Single-center | Germany | No | 2001–2016 | 242 | 126 |
| Fukui et al. | Single-center | Japan | No | 2006–2013 | 259 | 245 |
| Chen et al. | Multi-center, Taiwan NHIRD | Taiwan | No | 2004–2013 | 2,883 | 1,286 |
| Santamaria et al. | Single-center | Italy | No | 2009–2016 | 98 | 36 |
| Hirata et al. | Single-center | Japan | No | 2009–2013 | 37 | 48 |
IRAD, International Registry of Acute Aortic Dissections; STS, Society of Thoracic Surgeons; NHIRD, National Health Insurance Research Database.
The NOS evaluation is displayed in Table 2. Nine of 16 studies included were scored eight or nine points suggesting they have good quality (9,11,17,19,26-29,32). Three studies scored less than six points which was due to no description of comparability of cohorts, or lack of information about the method for outcomes ascertainment and assessment (10,30,33). The 12 studies scored as having moderate to good quality (NOS scores ≥6) included a total of 6,947 men and 3,707 women.
Table 2
| Studies | Selection (maximum four stars) |
Comparability (maximum two stars) |
Outcome (maximum three stars) |
Total |
|---|---|---|---|---|
| Gasser et al. | *** | – | ** | 5 |
| Huckaby et al. | *** | ** | *** | 8 |
| Liu et al. | ** | ** | * | 5 |
| Li et al. | *** | ** | *** | 8 |
| Norton et al. | *** | ** | *** | 8 |
| Rios et al. | ** | – | * | 3 |
| Sabashnikov et al. | ** | ** | ** | 6 |
| Suzuki et al. | *** | ** | *** | 8 |
| Yousef et al. | *** | ** | *** | 8 |
| Conway et al. | *** | – | *** | 6 |
| Friedrich et al. | *** | ** | *** | 8 |
| Fukui et al. | **** | ** | *** | 9 |
| Chen et al. | *** | ** | *** | 8 |
| Santamaria et al. | *** | ** | *** | 8 |
| Hirata et al. | ** | * | ** | 5 |
Total scores greater or equal to six were considered moderate to good quality. NOS, Newcastle-Ottawa Scale.
Patient and operative characteristics
Table 3 summarizes pooled number of studies and patients analyzed as well as results for preoperative patient characteristics, intraoperative variables, and perioperative outcomes from the included studies (see Appendix 3, Figures S1-S24). Women were older than men [mean difference, 8.07 years; 95% confidence interval (CI): 7.05, 9.10; P<0.001; ten studies included (10,303 patients)] and had a lower body mass index [mean difference, −1.09; 95% CI: −1.56, −0.62; P<0.001; seven studies (5,379 patients)]; these analyses showed moderate heterogeneity (I2=62% and I2=54%, respectively). Women had higher rates of hypertension [risk ratio (RR), 1.06; 95% CI: 1.03, 1.09; P<0.001; ten studies (10,303 patients)] and lower rates of chronic kidney disease [RR, 0.74; 95% CI: 0.59, 0.93; P=0.01; seven studies (9,154 patients)]; these analyses showed moderate heterogeneity (I2=26% and I2=38%, respectively). Women were less likely to be currently smoking [RR, 0.47; 95% CI: 0.25, 0.88; P=0.02; five studies (4,144 patients)]; though this analysis showed high heterogeneity (I2=94%). Women were also less likely to have undergone previous cardiovascular surgery [RR, 0.70; 95% CI: 0.59, 0.84; P<0.001; six studies (9,005 patients)]; this analysis showed low heterogeneity (I2=0%).
Table 3
| Variables | Number studies | Male, n | Female, n | RR/MD | 95% CI | P value | I2 (%) |
|---|---|---|---|---|---|---|---|
| Preoperative variables† | |||||||
| Age | 10 | 6,792 | 3,511 | 8.07 | 7.05, 9.10 | <0.001* | 62 |
| BMI | 7 | 3,478 | 1,901 | −1.09 | −1.56, −0.62 | <0.001* | 54 |
| Hypertension | 10 | 6,792 | 3,511 | 1.06 | 1.03, 1.09 | <0.001* | 26 |
| Prior CVA | 4 | 3,767 | 1,697 | 1.78 | 1.01, 3.15 | 0.05 | 76 |
| Prior CKD | 7 | 6,093 | 3,061 | 0.74 | 0.59, 0.93 | 0.01* | 38 |
| CTD | 7 | 6,106 | 3,105 | 1.17 | 0.91, 1.50 | 0.23 | 0 |
| CAD | 5 | 1,468 | 785 | 0.94 | 0.77, 1.15 | 0.57 | 0 |
| COPD | 6 | 4,165 | 1,970 | 1.05 | 0.82, 1.34 | 0.71 | 20 |
| Diabetes mellitus | 9 | 6,524 | 3,385 | 1.22 | 0.94, 1.59 | 0.13 | 62 |
| Current smoker | 5 | 2,696 | 1,448 | 0.47 | 0.25, 0.88 | 0.02* | 94 |
| Prior cardiac surgery | 6 | 6,052 | 2,953 | 0.70 | 0.59, 0.84 | <0.001* | 0 |
| Shock | 5 | 2,955 | 1,693 | 1.13 | 0.99, 1.29 | 0.07 | 0 |
| Malperfusion | 5 | 1,382 | 806 | 0.94 | 0.81, 1.10 | 0.46 | 21 |
| Intraoperative variables‡ | |||||||
| CPB time | 9 | 3,740 | 2,163 | −11.51 | −20.58, −2.44 | 0.01* | 86 |
| Nadir temperature | 3 | 2,566 | 1,301 | 0.27 | −0.05, 0.59 | 0.10 | 0 |
| AVR | 6 | 3,335 | 1,882 | 0.80 | 0.70, 0.90 | <0.001* | 0 |
| Total arch | 9 | 4,018 | 2,400 | 0.73 | 0.58, 0.93 | 0.01* | 85 |
| Hemiarch | 5 | 2,987 | 1,641 | 1.06 | 1.01, 1.11 | 0.01* | 11 |
| Cannulation | |||||||
| Aortic | 4 | 2,610 | 1,422 | 1.17 | 0.93, 1.48 | 0.18 | 73 |
| Axillary/subclavian | 6 | 3,050 | 1,627 | 0.83 | 0.77, 0.91 | <0.001* | 0 |
| Femoral | 6 | 3,050 | 1,627 | 0.98 | 0.89, 1.08 | 0.68 | 1 |
| Perioperative outcomes | |||||||
| In-hospital/30-day mortality | 11 | 6,972 | 3,757 | 1.18 | 0.96, 1.45 | 0.12 | 46 |
| Stroke | 10 | 6,901 | 3,686 | 1.07 | 0.90, 1.28 | 0.46 | 12 |
| Reoperation for bleeding | 8 | 3,588 | 2,100 | 0.90 | 0.75, 1.09 | 0.28 | 0 |
†, analysis of preoperative variables used unmatched data from Sabashnikov et al. (34) and Li et al. (11); ‡, analysis of intraoperative variables used unmatched data from Sabashnikov et al. (34); *, P<0.05. RR, risk ratio; MD, mean difference; CI, confidence interval; BMI, body mass index; CVA, cerebrovascular accident; CKD, chronic kidney disease; CTD, connective tissue disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; AVR, aortic valve replacement.
Intraoperatively, females had shorter cardiopulmonary bypass times [mean difference, −11.51 minutes; 95% CI: −20.58, −2.44; P=0.01; nine studies (5,903 patients)]; however, this analysis showed high heterogeneity (I2=86%). Furthermore, women had less extensive surgery with lower rates of total arch replacements [RR, 0.73; 95% CI: 0.58, 0.93; P=0.01; nine studies (6,418 patients)]; though this analysis showed high heterogeneity (I2=85%). Women received more hemiarch replacements [RR, 1.06; 95% CI: 1.01, 1.11; P=0.01; five studies (4,628 patients)] and less aortic valve replacements [RR, 0.80; 95% CI: 0.70, 0.90; P<0.001; six studies (5,217 patients)]; these analyses showed low heterogeneity (I2=11% and I2=0%, respectively). Cannulation strategy was also different between the sexes with women receiving axillary cannulation less frequently than men [RR, 0.83; 95% CI: 0.77, 0.91; P<0.001; six studies (4,677 patients)], though rates of femoral cannulation did not differ significantly [RR, 0.98; 95% CI: 0.89, 1.08; P=0.68; six studies (4,677 patients)]; these analyses showed low heterogeneity (I2=0% and I2=1%, respectively). No other sex differences in preoperative and intraoperative variables were noted.
For perioperative outcomes, the aggregate studies showed no differences in in-hospital/30-day mortality, perioperative stroke, or early reoperation for bleeding.
In analyses where ≥10 studies were included (i.e., age, hypertension, in-hospital/30-day mortality, and perioperative stroke) we did not note any large asymmetry in funnel plots (Appendix 4, Figures S25-S28) and detected no significant asymmetry in funnel plots by Egger’s test for age (P=0.92), hypertension (P=0.35), in-hospital/30-day mortality (P=0.84), and perioperative stroke (P=0.20).
Long-term survival
Overall survival (Figure 2A) was significantly worse in women in comparison to men (P<0.001). Using the pooled Kaplan-Meier curves, estimates for 5-year survival were 82.4% in men and 78.1% in women, and for 10-year survival were 68.1% in men and 63.4% in women. Sensitivity analysis showed long-term survival remained poorer for women after removing studies with propensity-matched cohorts (P=0.0004) and studies graded as poor quality (P=0.002). Landmark analysis of patients surviving 1-year post-surgery (Figure 2B) also showed poorer survival for women in the long-term (P=0.014), though the difference between the sexes was less with 5-year survival of 90.9% in males vs. 88.9% in females and a 10-year survival of 75.1% in males vs. 72.0% in females. For reference, the long-term survival curves were superimposed with the expected survival of age- and sex-matched Canadians (Figure 2C).

Of the two studies that did not publish Kaplan-Meier curves for survival, Chen and colleagues reported no significant differences between male and female patients in all-cause mortality [35.3% vs. 34.7%; odds ratio (OR), 0.99; 95% CI: 0.88, 1.10] across a mean follow up of 2.8±2.7 years (17). Meanwhile, Santamaria and colleagues reported worse long-term survival in female patients (47% vs. 24%, P=0.005) across a mean follow-up of 3.25±3 years (29).
Long-term reoperation
Long-term freedom from reoperation (Figure 3) was lower in men compared to women (P<0.001). Freedom from reoperation at 5-year was estimated to be 88.4% in men vs. 93.1% in women. Sensitivity analysis was not performed for this outcome, as none of the included studies had propensity-matched cohorts and all studies received scores ≥6 on NOS assessment. Although Gasser and colleagues did not publish a Kaplan-Meier curve for freedom from reoperation, they reported higher rates of reoperation during follow-up for men (8.6% vs. 4.0%) and this difference was not statistically significant (P=0.08) (10).

Discussion
This meta-analysis evaluated the influence of sex on long-term death and reoperation following surgical treatment of ATAAD using all available updated sex-stratified data. Our literature search identified 15 studies reporting sex-specific data for long-term survival and/or reoperation which included over 11,000 ATAAD patients undergoing surgical repair. We found that men and women now have comparable perioperative outcomes. However, women had worse long-term survival, and men had higher rates of long-term reoperation.
Our study found that women presented on average ~8 years older than men, which is similar to the results reported by two previous meta-analyses (15,20). Furthermore, we found that men received more extensive surgical repair for ATAAD which could potentially be due to more extensive distal disease involving the descending thoracic and abdominal aorta in men (36). Despite similar rates of preoperative malperfusion syndrome, there was a trend towards women presenting with shock, which may have influenced the surgeon’s decision to perform a more conservative intervention.
Our study was the first to meta-analyze sex differences in total arch and hemiarch replacements, and arterial cannulation. We found that women received less total arch replacements and more hemiarch replacements. Furthermore, women were less likely to receive axillary cannulation, though there were no sex differences in rates of direct aortic and femoral cannulation. Ohira and colleagues also found that female sex was an independent predictor of axillary non-cannulation and hypothesized this could be due to a combination of lower vessel caliber, increased age, and increased urgency of surgery which prohibited against the typically longer times required to establish axillary cannulation (37). While some studies have found comparable outcomes across all three cannulation strategies (38), two meta-analyses showed axillary cannulation is superior to femoral cannulation for in-hospital mortality and stroke (39,40).
The finding that there were no sex differences in the rates of in-hospital/30-day mortality and perioperative stroke in our analysis was similar to recent reports and meta-analyses (15,16,20). Previously, there was a gender gap reported in perioperative outcomes which has been eliminated over time. Contemporary data from IRAD shows female in-hospital mortality following ATAAD surgery decreased from 27% to 16% (trend P=0.114), and female sex was eliminated as an independent predictor of mortality when considering patients enrolled in the last decade of the study [2006–2017] (9). Moreover, CTAC data also shows equalization between the sexes driven largely by significant improvements for women undergoing urgent aortic arch surgery (30% to 11%, trend P=0.01) (14). This era effect may be due to advances in surgical techniques and improving safety of aortic surgery, which could drive large improvements in outcomes for female patients due to the initial disparities between the sexes. Our updated meta-analysis of all available data, including more contemporary results, can make this effect more definitive.
Despite having comparable in-hospital/30-day mortality, long-term survival was worse in women. One important factor influencing this could be the older age at presentation of women compared to men. Across included studies, the average age at presentation was 65 years for women compared to 57 years for men. In Canada, the general population life expectancy was 22.2 years for women aged 65 and 26.1 years for men aged 57 years (25). Similarly, in the United States, life expectancy was 20.8 years for women aged 65 and 24.2 years for men aged 57 (41). However, the differences in yearly survival between the average Canadian 65-year-old woman and 57-year-old man were smaller than the differences in survival between the sexes following surgery for ATAAD (shown in Figure 2C). This suggests that older age at presentation for female patients is an important factor though may not fully account for the sex-based differences in long-term survival.
Furthermore, in our pooled Kaplan-Meier plot, the sex-specific survival curves demonstrated the greatest separation within the first-year post-surgery. Given the dynamic changes in the outcome gap between men and women within the early perioperative period over the past 10–20 years, we sought to neutralize this confounding effect. Therefore, we carried out a landmark analysis of patients alive at 1 year, which showed a smaller difference between men and women, though survival remained significantly poorer in women. In their meta-analysis, Carbone and colleagues found higher risk of mortality at 5- and 10-year for women, though their study did not estimate pooled survival rates at 5- and 10-year (20). Our findings are in contrast to the meta-analysis by Meccanici and colleagues who reported comparable long-term survival between men and women, however their analysis only included five studies and did not analyze differences beyond 5-year post-surgery (15). Overall, the sum of the data suggests that a difference in long-term survival between the sexes after surgery for ATAAD does exist but is not large. Encouragingly, our analysis suggests that this difference is at least partially accounted for by the expected differences in long-term survival of age- and sex-matched cohorts in the general population.
Despite men having more extensive surgery, there was a higher rate of reoperation among them. It has been suggested that this could be due to lower age at presentation, as advanced age may be a reason against performing late reoperation (19,28). Other confounders could be the higher long-term mortality in females, which is a competing risk. Geirsson and colleagues reported more extensive distal dissection and younger age, both more common in men, to be independent predictors of distal reoperations in surgically treated ATAAD patients (42), while An and colleagues also reported advanced age and female sex as protective factors against reoperation amongst patients who survived at least 90 days post-surgery (18). The only previous study to perform meta-analysis of long-term reoperation is Meccanici and colleagues, which included only two studies (15). Nonetheless, they also reported higher rates of reoperation in men.
Limitations
There are several limitations to our current study. Firstly, this is a systematic review of retrospective, observational studies. The majority of included studies have provided data regarding sex differences in unmatched cohorts. However, two included studies provided outcomes data from propensity-matched cohorts (11,34); we performed sensitivity analysis on the effect of including these two propensity-matched cohorts in our meta-analysis and found no significant changes to our results. Secondly, our inclusion criteria limited our analysis to studies providing long-term sex-specific outcomes in surgically treated ATAAD patients. As such, several studies included in previous meta-analyses were excluded from the present analysis. Of these, the most significant exclusion is sex-stratified data from the large, multicenter, international German Registry for Acute Aortic Dissection Type A (GERAADA) which has reported on sex differences in preoperative characteristics, intraoperative variables, and perioperative outcomes, however has not yet published data on long-term follow-up (36). Thirdly, some variables showed high heterogeneity (Table 3), including prior cerebrovascular accident and current smoking, which may be due to challenges in the classification and reporting of these variables, as well as total arch and aortic cannulation, which may be due to differences in standard practice between institutions. Finally, there are limitations to the algorithm used to reconstruct individual patient-level data and generate pooled Kaplan-Meier curves. The algorithm assumes a constant rate of censoring over time (22), and some individual studies included in our analysis capture mortality as a censored event when the outcome of interest is reoperation, as such the cumulative probability of reported reoperation may be susceptible to inaccuracy. Nonetheless, our estimated 5- and 10-year reoperation rates were similar to those reported in individual studies.
Conclusions
In this updated meta-analysis of surgically treated ATAAD patients, sex differences in preoperative characteristics and intraoperative management were apparent. Women had worse long-term survival compared to men. This difference remains even amongst patients who survive the first-year post-surgery, though the difference is smaller. Male sex was associated with higher rates of reoperation. Future work is needed to understand if there are any associations between specific preoperative and intraoperative variables and long-term outcomes.
Acknowledgments
The authors thank Ashley Farrell (Library and Information Services, University Health Network, Toronto, ON, Canada) for research librarian support in reviewing and suggesting improvements for the search strategy.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Yamasaki M, Yoshino H, Kunihara T, et al. Risk analysis for early mortality in emergency acute type A aortic dissection surgery: experience of Tokyo Acute Aortic Super-network. Eur J Cardiothorac Surg 2021;60:957-64. [Crossref] [PubMed]
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112-22. [Crossref] [PubMed]
- Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation 2014;130:S45-50. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation 2004;109:3014-21. [Crossref] [PubMed]
- Smedberg C, Steuer J, Leander K, et al. Sex differences and temporal trends in aortic dissection: a population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur Heart J 2020;41:2430-8. [Crossref] [PubMed]
- Huckaby LV, Sultan I, Trimarchi S, et al. Sex-Based Aortic Dissection Outcomes From the International Registry of Acute Aortic Dissection. Ann Thorac Surg 2022;113:498-505. [Crossref] [PubMed]
- Gasser S, Stastny L, Kofler M, et al. Type A aortic dissection is more aggressive in women. Eur J Cardiothorac Surg 2022;62:ezac040. [Crossref] [PubMed]
- Li Y, Yang N, Liu S, et al. Sex Differences of Clinical Presentation and Outcomes in Propensity-Matched Patients with Acute Type A Aortic Dissection. Heart Surg Forum 2021;24:E311-6. [Crossref] [PubMed]
- Chemtob RA, Hjortdal V, Ahlsson A, et al. Effects of Sex on Early Outcome following Repair of Acute Type A Aortic Dissection: Results from The Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD). Aorta (Stamford) 2019;7:7-14. [Crossref] [PubMed]
- Chung J, Stevens LM, Ouzounian M, et al. Sex-Related Differences in Patients Undergoing Thoracic Aortic Surgery. Circulation 2019;139:1177-84. [Crossref] [PubMed]
- Chung JCY, Bhatt N, Stevens LM, et al. Trends in sex-specific differences following aortic arch repair: results from the Canadian Thoracic Aortic Collaborative. Ann Cardiothorac Surg 2023; [Crossref]
- Meccanici F, Gökalp AL, Thijssen CGE, et al. Male-female differences in acute thoracic aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2022;34:616-27. [Crossref] [PubMed]
- Lawrence KW, Yin K, Connelly HL, et al. Sex-based outcomes in surgical repair of acute type A aortic dissection: A meta-analysis and meta-regression. J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref]
- Chen FT, Chou AH, Chan YH, et al. Sex-related differences on the risks of in-hospital and late outcomes after acute aortic dissection: A nationwide population-based cohort study. PLoS One 2022;17:e0263717. [Crossref] [PubMed]
- An KR, de Mestral C, Tam DY, et al. Surveillance Imaging Following Acute Type A Aortic Dissection. J Am Coll Cardiol 2021;78:1863-71. [Crossref] [PubMed]
- Suzuki T, Asai T, Kinoshita T. Clinical differences between men and women undergoing surgery for acute Type A aortic dissection. Interact Cardiovasc Thorac Surg 2018;26:944-50.
- Carbone A, Ranieri B, Castaldo R, et al. Sex differences in type A acute aortic dissection: a systematic review and meta-analysis. Eur J Prev Cardiol 2023;30:1074-89. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Statistics Canada. Life expectancy and other elements of the complete life table, three-year estimates, Canada, all provinces except Prince Edward Island. 2022. Available online: https://doi.org/
10.25318/1310011401-eng - Yousef S, Navid F, Zhu J, et al. Sex-based outcomes after surgery for acute type A aortic dissection. J Card Surg 2022;37:4342-7. [Crossref] [PubMed]
- Friedrich C, Salem MA, Puehler T, et al. Sex-specific risk factors for early mortality and survival after surgery of acute aortic dissection type a: a retrospective observational study. J Cardiothorac Surg 2020;15:145. [Crossref] [PubMed]
- Fukui T, Tabata M, Morita S, et al. Gender differences in patients undergoing surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:581-7.e1. [Crossref] [PubMed]
- Santamaria V, Schirone L, Vinciguerra M, et al. Predictors for outcome in type A aortic dissection: A focus on false lumen. Cirugía Cardiovascular 2021;28:71-6.
- Hirata K, Oda S, Suzuki R, et al. Long-term prognostic value of the combined assessment of clinical and computed tomography findings in type: An acute aortic dissection. Medicine (Baltimore) 2020;99:e23008. [Crossref] [PubMed]
- Liu YJ, Wang XZ, Wang Y, et al. Correlation between Sex and Prognosis of Acute Aortic Dissection in the Chinese Population. Chin Med J (Engl) 2018;131:1430-5. [Crossref] [PubMed]
- Norton EL, Kim KM, Fukuhara S, et al. Differences among sexes in presentation and outcomes in acute type A aortic dissection repair. J Thorac Cardiovasc Surg 2023;165:972-81. [Crossref] [PubMed]
- Rios F, Perez D, Soca G, et al. Predictive Factors of Mortality in Acute Aortic Dissection and Validity of the EuroSCORE Algorithm in a Small-Sized Cardiac Surgery Institution. Braz J Cardiovasc Surg 2020;35:878-83. [Crossref] [PubMed]
- Sabashnikov A, Heinen S, Deppe AC, et al. Impact of gender on long-term outcomes after surgical repair for acute Stanford A aortic dissection: a propensity score matched analysis. Interact Cardiovasc Thorac Surg 2017;24:702-7. [Crossref] [PubMed]
- Conway BD, Stamou SC, Kouchoukos NT, et al. Effects of Gender on Outcomes and Survival Following Repair of Acute Type A Aortic Dissection. Int J Angiol 2015;24:93-8. [Crossref] [PubMed]
- Rylski B, Georgieva N, Beyersdorf F, et al. Gender-related differences in patients with acute aortic dissection type A. J Thorac Cardiovasc Surg 2021;162:528-535.e1. [Crossref] [PubMed]
- Ohira S, Malekan R, Kai M, et al. Direct Axillary Artery Cannulation for Type A Dissection and Impact of Dissected Innominate Artery. Ann Thorac Surg 2022;113:1183-90. [Crossref] [PubMed]
- Yousef S, Brown JA, Serna-Gallegos D, et al. Central versus peripheral cannulation for acute type A aortic dissection. J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref]
- Benedetto U, Mohamed H, Vitulli P, et al. Axillary versus femoral arterial cannulation in type A acute aortic dissection: evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg 2015;48:953-9. [Crossref] [PubMed]
- Ren Z, Wang Z, Hu R, et al. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur J Cardiothorac Surg 2015;47:408-15. [Crossref] [PubMed]
- Arias E, Xu J. United States life tables, 2020. 2022. Available online: https://dx.doi.org/
10.15620/cdc:118055 - Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64. [Crossref] [PubMed]






