Prognostic implication of left atrial strain in patients experiencing early recurrence of atrial fibrillation after totally thoracoscopic ablation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, resulting in increased mortality, morbidity, and poorer quality of life (1,2). For patients who have symptomatic AF refractory to medical therapy, catheter or surgical ablation has emerged as an effective option for rhythm control (3-5). Early recurrence (ER) after ablation therapy has been recognized. Reportedly, as many as 61% of patients experience ER of arrhythmia (6-10). As ER is believed to be benign and related to transient local inflammatory and pro-arrhythmic states, reintervention is not recommended during the “blanking period” of the initial 3 months after ablation (4). However, increasing evidence suggests that patients with ER have a higher risk of late recurrence (6,8,10-12). While various predictors of ER after ablation have been proposed, such as left atrial (LA) enlargement, non-paroxysmal AF, or comorbidities (13,14), limited data exists on predictors of late recurrence in patients who experience ERs. Therefore, identifying predictors of late recurrence in patients who experience ERs is crucial for improving patient outcomes. Totally thoracoscopic ablation (TTA) has recently been highlighted as an effective option with minimal invasiveness. Although favorable outcomes of TTA have been reported (15-17), recurrence after a successful initial procedure remains problematic. However, in most studies, the blanking period was excluded from the definition of AF recurrence (15,17-19). As ER after Maze procedure is a predictor of late failure (20-22), ER after TTA may be clinically significant. To date, the frequency and prognosis of ER after TTA for AF are unknown. In this study, we investigate the clinical significance of early AF events during the blanking period and the role of preoperative LA strain in predicting late recurrence.
Methods
Study population
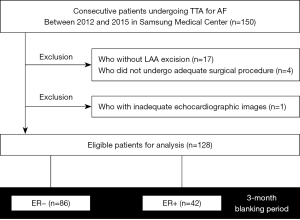
Between February 2012 and March 2015, patients undergoing TTA were enrolled in a prospective registry in the Samsung Medical Center, Republic of Korea. Patients with AF refractory to at least 1 antiarrhythmic drug or electrical cardioversion were candidates for TTA. The indications for TTA also included refractory symptoms, history of stroke, or intolerance of anticoagulant therapy. TTA was contraindicated in patients with LA thrombi or intolerance of one-lung ventilation. Among the 150 consecutive patients who underwent TTA during the study period, three patients who did not undergo an adequate surgical procedure due to a sizable LA or severe pericardial adhesion, one patient who underwent LA appendage (LAA) resection alone for stroke prevention, 17 patients who did not undergo LAA resection during the surgery, and one patient who did not have adequate echocardiographic images for strain measurement were excluded; the final analysis included 128 patients (Figure 1). The Samsung Medical Center Institutional Review Board approved the present study (No. 2020-05-146-003).
Thoracoscopic procedures
TTA is a video-assisted thoracoscopic surgical technique without thoracotomy and cardiopulmonary bypass. A bilateral approach is required and the detailed techniques of TTA have been described in our previous report (23). Ablation lines for pulmonary vein isolation were created using an AtriCure Isolator Transpolar Clamp (AtriCure, Inc., Cincinnati, OH, USA) and the LA roof and floor lesions connecting both pulmonary veins were drawn with a linear pen device (AtriCure, Inc.). After creating pulmonary vein isolation and box lesions, exit and entrance block tests using an AtriCure Cooltip pen (AtriCure, Inc.) were performed. Next, the ganglionated plexuses were examined and ablated. The ligament of Marshall, which might be a source of adrenergic atrial tachycardia, was always divided and ablated. The LAA was removed by stapling with an Echelon Flex 60 articulating endoscopic linear stapler (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA).
Postoperative care and endpoint
Patients were monitored in the intensive care unit for the first 24 hours. Heparin was administered after 2 hours postoperatively, and patients were switched to either warfarin or a direct-acting oral anticoagulant as soon as possible. Oral amiodarone was prescribed if the heart rate was >80 beats/min with AF rhythm at rest. Patients were followed up at 3, 6, and 12 months, and annually thereafter with 24-hour Holter monitoring. Antiarrhythmic drugs were discontinued after 3 months or up to 6 months based on the 24-hour Holter monitoring results.
Anticoagulants were also discontinued after 3 months based on the risk of stroke for each patient. ER was defined as any AF or atrial flutter (AFL) detected on electrocardiogram or lasting more than 30 seconds in 24-hour Holter monitoring during the blanking period (initial 3 months after TTA). If hybrid (planned) radiofrequency ablation was performed, the blanking period began after the last procedure. ERs were managed using electrical cardioversion or antiarrhythmic drugs. AF or AFL events after the blanking period were defined as late recurrence. Patients were followed up for 5 years after TTA and the median follow-up duration of study subjects was 5.0 years.
Echocardiography and speckle tracking imaging
Preoperative comprehensive transthoracic echocardiography was performed with commercially available equipment (Vivid 7, GE Medical Systems, Milwaukee, WI, USA, Acuson Sequoia 512, Siemens Medical Solution, Mountain View, CA, USA, or Sonos 5500, Philips Medical System, Andover, MA, USA) according to practice guidelines (24). Left ventricular (LV) end-diastolic and end-systolic diameter as well as LA diameter were calculated from parasternal long-axis view. LV ejection fraction was calculated from two-dimensional recordings using the modified biplane Simpson’s method. LA volume was assessed using the modified biplane area-length method and indexed to body surface area (LA volume index, LAVI). Early diastolic mitral inflow velocity (E) was measured using the pulsed wave Doppler method by placing the sample volume at the level of the mitral valve leaflet tips. The tissue Doppler-derived early diastolic mitral annular velocity (e’) was measured from the septal corner of the mitral annulus in the apical four-chamber view. For patients with AF rhythm, the average of five consecutive Doppler signals was used.
Peak longitudinal LA strain (reservoir strain) was measured using vendor-independent dedicated software (2D cardiac Performance Analysis 1.4, TomTec Imaging Systems, GmbH, Unterschleißheim, Germany) according to the current guideline (Figure S1) (25). Peak positive strain rate was calculated during LV systole. The LA endocardial border was automatically traced by the software and then manually adjusted in both apical four- and two-chamber views. Pulmonary veins and LAA orifices were carefully excluded. The regions of interest encompassed the endocardial border of the mitral annulus, and the thickness of regions of interest was adjusted to the thinnest part to adapt to the atria. Two independent echocardiologists blinded to clinical status analyzed the data. All strain values were calculated from cardiac cycle with heart rate <110 beats per minute to avoid inadequate LA emptying or filling. LA stiffness index was defined as E/e’ divided by peak longitudinal LA strain (26).
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median [interquartile range (IQR)]. Categorical variables were compared using the chi-square test. Continuous variables were compared using the Student’s t-test or Mann-Whitney U test as appropriate. The incidence of recurrent AF or AFL at 5 years after TTA were estimated using the Kaplan-Meier method. Survival curves were compared with the log-rank test. Independent predictors for 5-year AF or AFL recurrence were analyzed using the univariable and multivariable Cox regression models.
Variables with P value <0.2 between patients with and without 5-year AF or AFL recurrence (LAVI, LA strain, and LAA fibrosis area) or clinically relevant variables (age, sex, paroxysmal AF, and radiofrequency catheter ablation) were included in the multivariable Cox regression model. The best cutoff value of LA strain to maximize the difference in 5-year recurrence rate was estimated by plotting the standardized log-rank statistic. All tests were two-sided and a P value <0.05 was considered statistically significant. Statistical analysis was performed using R 3.6.2 (R Foundation for Statistical Computing, https://www.r-project.org/).
Results
Baseline characteristics
A total of 128 patients were eligible for analysis (Figure 1). The mean age was 54.3±8.8 years, 95.3% were male, and 18.8% had paroxysmal AF (Table 1). The median LA diameter, LAVI, and LA strain were 45.0 mm (IQR, 40.0–50.0 mm), 45.2 mL/m2 (IQR, 36.4–54.8 mL/m2), and 15.3% (IQR, 12.1–19.2%), respectively. Among the 128 patients, 42 (32.8%) experienced ER during the blanking period. The median time to ER was 16.5 days (IQR, 8.0–34.5 days). Patients with ER had a significantly larger LAVI compared with those without ER (51.2 vs. 42.7 mL/m2). Other clinical or echocardiographic variables were not significantly different between patients with and without ER. Operative characteristics based on ER are presented in Table S1.
Table 1
| Variables | Overall (n=128) | ER (+) (n=42) | ER (−) (n=86) | P value |
|---|---|---|---|---|
| Clinical | ||||
| Age | 54.3±8.8 | 54.5±8.5 | 54.2±8.9 | 0.861 |
| Body mass index, kg/m2 | 25.2±2.8 | 25.0±3.0 | 25.1±2.2 | 0.893 |
| Male | 122 (95.3) | 39 (92.9) | 83 (96.5) | 0.636 |
| Hypertension | 49 (38.3) | 18 (42.9) | 31 (36.0) | 0.582 |
| Diabetes | 12 (9.4) | 2 (4.8) | 10 (11.6) | 0.353 |
| Previous stroke | 19 (14.8) | 5 (11.9) | 14 (16.3) | 0.697 |
| Paroxysmal AF | 24 (18.8) | 5 (11.9) | 19 (22.1) | 0.252 |
| CHADS2 score | 1.0 [0.0–2.0] | 1.0 [0.0–1.0] | 1.0 [0.0–1.0] | 0.860 |
| CHA2DS2 VASc score | 1.0 [0.0–2.0] | 1.0 [0.0–2.0] | 1.0 [0.0–2.0] | 0.633 |
| NT-proBNP, pg/mL | 254.8 [150.9–460.7] | 267.1 [175.7–435.0] | 253.0 [144.2–462.8] | 0.895 |
| Antiarrhythmic drugs before surgery | 96 (75.0) | 30 (71.4) | 66 (76.7) | 0.931 |
| Previous RFCA | 20 (15.6) | 3 (7.1) | 17 (19.8) | 0.112 |
| Hybrid RFCA | 91 (71.1) | 33 (78.6) | 58 (67.4) | 0.273 |
| Echocardiographic | ||||
| LVEDD, mm | 52.0 [49.0–54.5] | 52.0 [50.0–54.0] | 51.0 [49.0–55.0] | 0.748 |
| LVESD, mm | 32.5 [30.0–35.0] | 32.5 [30.0–35.0] | 32.5 [30.0–36.0] | 0.917 |
| LVEF, % | 60.0 [56.0–64.0] | 59.5 [56.0–64.0] | 60.0 [56.0–65.0] | 0.837 |
| E/e’ | 8.2 [6.3–10.1] | 8.3 [6.3–10.2] | 8.1 [6.3–10.0] | 0.847 |
| LAD, mm | 45.0 [40.0–50.0] | 46.0 [42.0–53.0] | 44.0 [40.0–49.0] | 0.119 |
| LAVI, mL/m2 | 45.2 [36.4–54.8] | 51.2 [41.7–59.6] | 42.7 [35.3–51.3] | 0.011 |
| LA strain, % | 15.3 [12.1–19.2] | 14.3 [11.8–17.0] | 16.2 [12.4–20.2] | 0.077 |
| Stiffness index, % | 0.5 [0.4–0.8] | 0.6 [0.4–0.8] | 0.5 [0.4–0.8] | 0.222 |
| Histologic | ||||
| LAA fibrosis area, % | 38.6 [33.1–44.7] | 38.2 [34.3–43.9] | 38.6 [32.8–46.7] | 0.945 |
Values are presented as mean ± SD, median [interquartile range] or n (%). ER, early recurrence; AF, atrial fibrillation; NT-proBNP, N-terminal-pro hormone B-type natriuretic peptide; LA, left atrial; RFCA, radiofrequency catheter ablation; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; LAVI, left atrial volume index; LAA, left atrial appendage.
Late recurrence in patients with and without ER
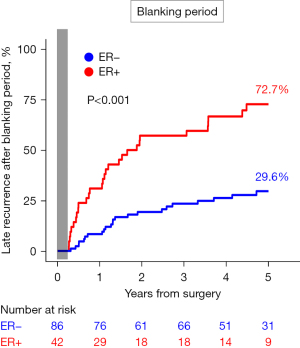
During the 5-year follow-up after TTA, patients with ER had a significantly higher risk of recurrent AF or AFL compared with those without ER [72.7% vs. 29.6%; hazard ratio (HR) =3.69, 95% confidence interval (CI): 2.14–6.36, P<0.001; Figure 2].
Predictors of late recurrence in patients with ER
Out of the 42 patients with ER, 30 (71.4%) had late recurrence of AF or AFL after the blanking period, while 12 (28.6%) did not have late recurrence. Table 2 shows baseline differences between patients with and without late recurrence. Among clinical, echocardiographic, and histologic variables, only LA strain showed a significant difference between patients with late recurrence and those without (median value, 13.2% vs. 17.8%, P=0.032). The best cutoff value of LA strain for predicting late recurrence was 18.6% (Figure S2 and Figure 3A). In multivariable Cox analysis, preoperative LA strain <18.6% was an independent predictor of late recurrence after the blanking period (adjusted HR =4.20, 95% CI: 1.08–16.29, P=0.038; Table 3).
Table 2
| Variables | Recurrence group (n=30) | Non-recurrence group (n=12) | P value |
|---|---|---|---|
| Clinical | |||
| Age | 55.0±8.4 | 53.2±9.0 | 0.546 |
| Body mass index, kg/m2 | 25.5±2.6 | 26.1±3.4 | 0.505 |
| Male | 27 (90.0) | 12 (100.0) | 0.636 |
| Hypertension | 13 (43.3) | 5 (41.7) | >0.999 |
| Diabetes | 1 (3.3) | 1 (8.3) | >0.999 |
| Previous stroke | 4 (13.3) | 1 (8.3) | >0.999 |
| Paroxysmal AF | 3 (10.0) | 2 (16.7) | 0.940 |
| CHADS2 score | 1.0 [0.0–1.0] | 0.5 [0.0–1.5] | 0.835 |
| CHA2DS2 VASc score | 1.0 [0.0–1.0] | 1.0 [0.0–1.5] | 0.859 |
| NT-proBNP, pg/mL | 280.6 [190.2–482.1] | 198.5 [151.8–344.2] | 0.208 |
| Antiarrhythmic drugs before surgery | 22 (73.3) | 8 (66.7) | 0.957 |
| Previous RFCA | 2 (6.7) | 1 (8.3) | >0.999 |
| Hybrid RFCA | 25 (83.3) | 8 (66.7) | 0.440 |
| Echocardiographic | |||
| LVEDD, mm | 53.0 [50.0–54.0] | 50.0 [47.5–55.0] | 0.219 |
| LVESD, mm | 32.5 [31.0–35.0] | 32.5 [29.5–35.0] | 0.634 |
| LVEF, % | 60.5 [56.0–64.0] | 57.5 [56.0–64.0] | 0.511 |
| E/e' | 8.8 [6.9–10.4] | 7.7 [6.0–8.8] | 0.252 |
| LAD, mm | 46.0 [42.0–54.0] | 46.5 [40.0–52.0] | 0.889 |
| LAVI, mL/m2 | 55.0 [41.0–62.0] | 47.8 [42.1–52.5] | 0.129 |
| LA strain, % | 13.2 [11.3–15.5] | 17.8 [13.9–20.5] | 0.032 |
| <18.6% | 27 (90.0) | 6 (50.0) | 0.015 |
| Stiffness index, % | 0.6 [0.5–0.8] | 0.4 [0.3–0.5] | 0.222 |
| Hybrid RFCA | 58 (67.4) | 33 (78.6) | 0.273 |
| Histologic | |||
| LAA fibrosis area, % | 38.5 [35.4–46.8] | 36.6 [31.2–41.8] | 0.179 |
Values are presented as mean ± SD, median [interquartile range] or n (%). ER, early recurrence; AF, atrial fibrillation; NT-proBNP, N-terminal pro-hormone B-type natriuretic peptide; RFCA, radiofrequency catheter ablation; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; LAVI, left atrial volume index; LA, left atrial; LAA, left atrial appendage.
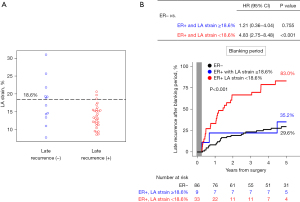
Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.01 (0.96–1.05) | 0.800 | |||
| Male | 0.68 (0.20–2.27) | 0.529 | |||
| Paroxysmal AF | 0.58 (0.18–1.93) | 0.376 | |||
| Pre or post RFCA | 1.10 (0.42–2.88) | 0.847 | |||
| LAVI, per 1 mL/m2 | 1.02 (0.99–1.05) | 0.309 | |||
| LA strain <18.6% | 4.10 (1.22–13.73) | 0.022 | 4.20 (1.08–16.29) | 0.038 | |
| LA appendage fibrosis, per 1% | 1.04 (0.99–1.09) | 0.135 | |||
All the listed variables were included in the multivariable Cox regression model. When a backward elimination method was used, only LA strain <18.6% remained in the model. AF, atrial fibrillation; ER, early recurrence; HR, hazard ratio; CI, confidence interval; LA, left atrial; LAVI, left atrial volume index.
Late recurrence based on LA strain
Among the patients with ER, those with LA strain <18.6% had a significantly higher risk of late recurrence compared to those without ER (83.0% vs. 29.6%, HR =4.83, 95% CI: 2.75–8.48, P<0.001). However, for patients with ER and LA strain ≥18.6%, the risk of late recurrence was similar to that of patients without ER (35.2% vs. 29.6%, HR =1.21, 95% CI: 0.36–4.04, P=0.755; Figure 3B).
Discussion
This is the first study to describe the prognostic implication of LA strain in patients with ER after TTA. The main findings of this study were as follows: the incidence of ER during the 3-month blanking period after TTA was 32.8%, patients with ER had a larger LAVI than those without ER, and preoperative LA strain was an independent predictor of late recurrence in patients with ER. Patients with ER but LA strain ≥18.6% had a late recurrence risk similar to those without ER. When energy is delivered to the myocardium to disrupt LA conduction, pathophysiological processes occur, such as coagulative necrosis, perfusion change due to artery trauma, thromboembolism, coronary spasm, oxidative stress, edema, or inflammation (27). These processes and electrical reconnection in LA contribute to arrhythmogenesis associated with ER after ablation. Early atrial arrhythmias during the blanking period, generally defined as the initial 3 months after ablation, are usually considered benign. While aggressive treatment for ER is not recommended because up to half of the patients with ER remain AF-free during long-term follow-up (4), identifying risk factors for ER is clinically important because observational studies have shown a relationship between ER and an increased risk of late recurrence (6,13,28,29). Previous studies have suggested that older age, male sex, larger LA, and non-paroxysmal AF are risk factors for developing ER after catheter ablation (7,29,30).
Thoracoscopic ablation was proposed as an effective rhythm control strategy due to the wide range of contiguous ablation and LAA exclusion that can be achieved through minimally invasive surgery. Observational studies and randomized trials have shown favorable outcomes of TTA (31-33). However, the frequency and clinical significance of ER after TTA has not been known, as previous studies excluded the blanking period when defining recurrent events (15,33,34). In the present study, we found that 32.8% of patients experienced ER within 3 months after TTA, and ER was associated with a 3.7-fold increase in the risk of late recurrence. These findings are consistent with studies on the Maze procedure. The reported incidence of ER within 3 months after Maze procedure has ranged from 28–49%, and it has been associated with more than 3-fold increase in late recurrence (20-22). Enlarged LA has consistently been identified as a risk factor for the development of ER (13,20,35). However, in previous studies on this issue, only LA diameter and not LAVI was reported as an echocardiographic parameter of LA remodeling (7,9-12,21). In the present study, we found that LAVI, and not LA diameter, was significantly higher in patients with ER. Although the LA anteroposterior dimension is the most widely used and reproducible measurement method, the assessment of LA size using only LA diameter assumes that all its dimensions change similarly when the LA enlarges, which is often not the case during LA remodeling (36). Therefore, the LAVI may provide more prognostic information than the LA diameter for predicting ER in patients undergoing thoracoscopic ablation.
Although the relationship between ER and an increased risk of late recurrence has been suggested, predictors of late recurrence in patients who experience ER after surgical ablation remain unknown. This topic has been investigated in several catheter ablation studies. For instance, a study by Jiang et al. reported that smaller LA size and lower P wave dispersion were predictors of delayed cure of ER (35). However, the delayed cure in that study was defined as maintenance of sinus rhythm for more than 2 months after ER, which did not represent long-term late recurrence. Tobacco use was also identified as a risk factor for late recurrence among patients with ER in another study (30), but its clinical usefulness is questionable. Additionally, the timing of ER, generally occurring after the first month of the blanking period, has been suggested to be a risk factor for late recurrence (8,29,37).
Advances in two-dimensional speckle-tracking software have enabled assessment of cardiac chamber deformation and myocardial function beyond the structural remodeling of the LA. LA strain has been studied extensively for its prognostic value in predicting successful electrical cardioversion (38) or catheter ablation (39). However, there has been relatively less research on the role of LA strain in surgical AF ablation. In the present study, preoperative peak LA strain emerged as an independent predictor of late recurrence in patients with ER. Among patients with LA strain ≥18.6%, ER did not pose a significant risk for late recurrence after the blanking period. In contrast, the risk of late recurrence after the blanking period was very high (83.0%) when patients with LA strain <18.6% experienced ER. In agreement with previous studies (30,35), LA enlargement was not found to be a significant predictor of late recurrence in patients with ER in the present study. These findings suggest that functional parameters, such as LA strain, may provide additional prognostic information for determining further treatment of ER following ablation.
The present study had several limitations. Firstly, this was a single-center retrospective study, which may limit the generalizability of the findings to other centers with different patient populations and physician experience with thoracoscopic ablation. Secondly, although annual Holter monitoring is recommended at our institution for all patients undergoing TTA, 24-hour Holter monitoring was not performed systematically in this study due to its observational nature. However, 84.4% of the patients (108 of 128) had undergone 24-hour Holter monitoring, with a median of four monitoring per patient, and the remaining patients had multiple electrocardiograms at each visit. Nonetheless, the possibility of asymptomatic paroxysmal AF cannot be ruled out completely. Thirdly, the best cutoff value of LA strain may vary depending on the quality of echocardiography images, the level of experience in delineating regions of interests, or speckle-tracking software used (25).
Nonetheless, this is the first study in which the frequency of ER after thoracoscopic ablation has been investigated. The results suggest that LA strain is a potential predictor of late recurrence in patients with ER following ablation therapy. Future large-scale studies are needed to confirm the current findings.
Conclusions
ER during the blanking period was a significant risk factor for late recurrence in patients undergoing TTA. LA strain was found to be an independent predictor of late recurrence in patients with ER.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke 2021;16:217-21. [Crossref] [PubMed]
- Aliot E, Botto GL, Crijns HJ, et al. Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace 2014;16:787-96. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. Erratum in: Eur Heart J 2021;42:507 Erratum in: Eur Heart J 2021;42:546-7. Erratum in: Eur Heart J 2021;42:4194. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-51. [Crossref] [PubMed]
- Steinberg C, Champagne J, Deyell MW, et al. Prevalence and outcome of early recurrence of atrial tachyarrhythmias in the Cryoballoon vs Irrigated Radiofrequency Catheter Ablation (CIRCA-DOSE) study. Heart Rhythm 2021;18:1463-70. [Crossref] [PubMed]
- Koyama T, Sekiguchi Y, Tada H, et al. Comparison of characteristics and significance of immediate versus early versus no recurrence of atrial fibrillation after catheter ablation. Am J Cardiol 2009;103:1249-54. [Crossref] [PubMed]
- Willems S, Khairy P, Andrade JG, et al. Redefining the Blanking Period After Catheter Ablation for Paroxysmal Atrial Fibrillation: Insights From the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ Arrhythm Electrophysiol 2016;9:e003909. [Crossref] [PubMed]
- Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol 2009;20:1089-94. [Crossref] [PubMed]
- Themistoclakis S, Schweikert RA, Saliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008;5:679-85. [Crossref] [PubMed]
- Liang JJ, Elafros MA, Chik WW, et al. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: Timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm 2015;12:2461-8. [Crossref] [PubMed]
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002;40:100-4. [Crossref] [PubMed]
- Kim YG, Boo KY, Choi JI, et al. Early Recurrence Is Reliable Predictor of Late Recurrence After Radiofrequency Catheter Ablation of Atrial Fibrillation. JACC Clin Electrophysiol 2021;7:343-51. [Crossref] [PubMed]
- Bordignon S, Barra S, Providencia R, et al. The blanking period after atrial fibrillation ablation: an European Heart Rhythm Association survey on contemporary definition and management. Europace 2022;24:1684-90. [Crossref] [PubMed]
- Haldar S, Khan HR, Boyalla V, et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur Heart J 2020;41:4471-80. [Crossref] [PubMed]
- Castellá M, Kotecha D, van Laar C, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace 2019;21:746-53. [Crossref] [PubMed]
- Adiyaman A, Buist TJ, Beukema RJ, et al. Randomized Controlled Trial of Surgical Versus Catheter Ablation for Paroxysmal and Early Persistent Atrial Fibrillation. Circ Arrhythm Electrophysiol 2018;11:e006182. [Crossref] [PubMed]
- Ma N, Lu R, Zhao D, et al. Left Atrial Appendage Fibrosis and 3-Year Clinical Outcomes in Atrial Fibrillation After Endoscopic Ablation: A Histologic Analysis. Ann Thorac Surg 2020;109:69-76. [Crossref] [PubMed]
- Vos LM, Bentala M, Geuzebroek GS, et al. Long-term outcome after totally thoracoscopic ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:40-5. [Crossref] [PubMed]
- Choi JH, Hwang KW, Jung SM, et al. Incidence and clinical impact of early recurrence of atrial tachyarrhythmia after surgical ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:2898-906. [Crossref] [PubMed]
- Maroto LC, Carnero M, Silva JA, et al. Early recurrence is a predictor of late failure in surgical ablation of atrial fibrillation. Interact Cardiovasc Thorac Surg 2011;12:681-6. [Crossref] [PubMed]
- Minami K, Kazawa M, Kakuta T, et al. Early Atrial Tachyarrhythmia Recurrence Predicts Late Atrial Tachyarrhythmia Recurrence After the Cryo-Maze Procedure- An Observational Study. Circ J 2022;87:76-83. [Crossref] [PubMed]
- On YK, Park KM, Jeong DS, et al. Electrophysiologic Results After Thoracoscopic Ablation for Chronic Atrial Fibrillation. Ann Thorac Surg 2015;100:1595-602; discussion 1602-3. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591-600. [Crossref] [PubMed]
- Shaikh AY, Maan A, Khan UA, et al. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovasc Ultrasound 2012;10:48. [Crossref] [PubMed]
- Gottlieb LA, Dekker LRC, Coronel R. The Blinding Period Following Ablation Therapy for Atrial Fibrillation: Proarrhythmic and Antiarrhythmic Pathophysiological Mechanisms. JACC Clin Electrophysiol 2021;7:416-30. [Crossref] [PubMed]
- Calkins H, Gache L, Frame D, et al. Predictive value of atrial fibrillation during the postradiofrequency ablation blanking period. Heart Rhythm 2021;18:366-73. [Crossref] [PubMed]
- Onishi N, Kaitani K, Nakagawa Y, et al. The association between late-phase early recurrence within the blanking period after atrial fibrillation catheter ablation and long-term recurrence: Insights from a large-scale multicenter study. Int J Cardiol 2021;341:39-45. [Crossref] [PubMed]
- Andrade JG, Khairy P, Macle L, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol 2014;7:69-75. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Elesin D, et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol 2013;24:1338-43. [Crossref] [PubMed]
- Vos LM, Kotecha D, Geuzebroek GSC, et al. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace 2018;20:1790-7. [Crossref] [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [Crossref] [PubMed]
- Sindby JE, Vadmann H, Lundbye-Christensen S, et al. Percutaneous versus thoracoscopic ablation of symptomatic paroxysmal atrial fibrillation: a randomised controlled trial - the FAST II study. J Cardiothorac Surg 2018;13:101. [Crossref] [PubMed]
- Jiang H, Lu Z, Lei H, et al. Predictors of early recurrence and delayed cure after segmental pulmonary vein isolation for paroxysmal atrial fibrillation without structural heart disease. J Interv Card Electrophysiol 2006;15:157-63. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Das M, Wynn GJ, Morgan M, et al. Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhythm Electrophysiol 2015;8:846-52. [Crossref] [PubMed]
- Costa C, González-Alujas T, Valente F, et al. Left atrial strain: a new predictor of thrombotic risk and successful electrical cardioversion. Echo Res Pract 2016;3:45-52. [Crossref] [PubMed]
- Tops LF, Delgado V, Bertini M, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2011;57:324-31. [Crossref] [PubMed]






