Outcomes of aortic root replacement in patients with Marfan syndrome: the role of valve-sparing and valve-replacing approaches
Introduction
Marfan syndrome (MFS) is a heritable disease that results from autosomal dominant mutations in the gene that encodes fibrillin-1 (FBN1). It has far-reaching effects on the skeletal, ocular, and cardiovascular systems (1). The signature manifestation of cardiovascular pathology in patients with MFS is a dilated aortic root (Figure 1), which commonly results in aneurysm, aortic valve regurgitation, and an increased risk of aortic dissection, rupture, and related death. Historically, this and other cardiovascular involvement resulted in a greatly reduced lifespan (2). The original approach for aortic root replacement (ARR) was introduced by Bentall and De Bono (3) in 1968 using a Teflon graft and Starr valve in a 33-year-old male with presumed MFS. Mechanical valve-replacing (VR) ARR, such as the Bentall procedure, typically necessitates a life-long regimen of anticoagulation to mitigate the risk of thromboembolism, exposing young MFS patients to substantial limitations due to the potential for bleeding complications. To conserve the native aortic valve, Yacoub (4) introduced the sinus remodeling approach to ARR, and several iterations by David and others that relied on sinus reimplantation soon followed (5-8). At the end of the 20th century, Gott and others (9) published a landmark multi-center report describing nearly the entirety of surgical experience in ARR in patients with MFS at the time. Although this work demonstrated the clear benefit of elective ARR in such patients, the role of valve-sparing (VS) approaches remained unclear.
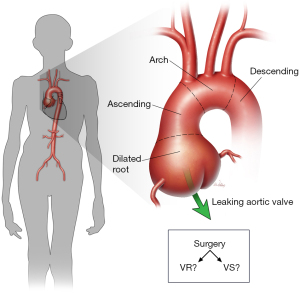
In contemporary repair, there remain two basic competing approaches to ARR in patients with MFS, VR and VS. In both of these broad approaches, several options have emerged over the last 40 years to reduce the risk of late complications and to capitalize on the development of new replacement materials (e.g., synthetic grafts with prefabricated sinuses and porcine aortic roots). Each option has advantages and disadvantages that help inform which approach to use in a given patient. An international multi-center prospective registry [Aortic Valve Operative Outcomes in Marfan Patients (AVOMP)] was initiated in 2005 to analyze clinical outcomes of ARR in MFS patients using either VR or VS techniques in hopes of better elucidating this choice of approach.
Overview of AVOMP
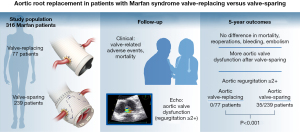
The AVOMP international observational study enrolled 316 participants from 19 surgical centers and followed them clinically, relying on echocardiographic surveillance by a core imaging center. Several publications have been released from this study, highlighting key findings and comparisons between the VR and VS groups. The initial publication in 2009 showed that early enrollment reflected the pervasive clinical trend towards using VS approaches and found no significant differences in valve-related or cardiac complications between the two groups (10). Additionally, an analysis in 2011 described intraoperative conversion from a VS to a VR procedure during the index surgery; importantly, intraoperative echocardiographic results may necessitate an unanticipated change in approach (11).
By 2014, initial follow-up was completed and analysis of early and 1-year results were presented. The VR group had older and sicker patients compared to the VS group. While there were no significant differences in survival, valve-related morbidity, or major adverse valve-related events (MAVRE) at 1 year, more bleeding events occurred after VR and more valve dysfunction after VS (12). In 2018, 3-year data were reported showing a higher incidence of aortic regurgitation ≥2+ (mild or greater) in the VS group compared to the VR group. Weighted Cox models revealed a higher risk of developing composite outcomes—MAVRE, valve-related morbidity, and structural valve deterioration/nonstructural valve dysfunction—in the VS group at 3 years (13). Notably, preoperative mitral regurgitation and urgent operations were identified as significant predictors of adverse outcomes. In 2023, the latest AVOMP publication presented 5-year results and continued to show that aortic regurgitation ≥2+ was more prevalent in the VS group (Figure 2) and contributed weighted Cox models to the evaluation of differences in composite events (namely, MAVRE, valve-related morbidity, and structural valve deterioration/nonstructural valve dysfunction) (14). Overall, the AVOMP study provides valuable insight into the outcomes of VR and VS procedures in patients with Marfan syndrome across several international centers. While both techniques are associated with low early complication rates, there are key differences in late outcomes, which should be considered when making clinical decisions. Because further long-term follow-up and analysis are necessary to fully understand the implications of these findings, the patients enrolled in the AVOMP study will be followed through 20 years.
Single-practice experience
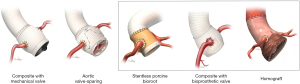
Although we serve as the coordinating center for the AVOMP study, our experience with ARR in patients with MFS predates this study and extends across four decades. Therefore, we present our related data stratified by the selection of a composite valve graft (CVG) using a mechanical valve, a VS approach, or the use of a bioprosthetic root (i.e., a homograft root, a porcine bioroot, or a specially prepared CVG using a tissue-based valve) (Figure 3).
Study protocol and patient cohort
Baylor College of Medicine’s institutional review board approved our clinical research protocol (#18095) in 2006. For patients who underwent operation after protocol approval, clinical data were collected prospectively, and informed consent was obtained whenever possible. A waiver of consent was approved for patients whose illness prevented them from providing consent and who had no family members available to provide consent for them. For patients who underwent surgery before the protocol was approved, waiver of consent was approved, and data were collected retrospectively from medical records. As necessary, medical records were reviewed to clarify abstracted data. From February 1991 to March 2023, 223 consecutive, elective ARRs in patients with MFS were performed by our practice at Baylor College of Medicine. Repairs included 113 (51%) using a mechanical CVG, 62 (28%) using a VS approach, and 48 (22%) using a bioprosthetic root. Fifteen of the 223 repairs we describe were included in the AVOMP registry.
Study definitions and follow-up
All data were collected by using standard definitions (15). All patients who were referred to our practice with the diagnosis of MFS and had elective ARR operations were included in the analysis (16). The proximal aorta included the aortic root, the ascending aorta, and the aortic arch. Aortic interventions included open and endovascular procedures performed on any aortic segment. We defined operative death as death within 30 days of surgery or before final discharge from our hospital or any other hospital or long-term acute care facility to which patients might have been transferred. Postoperative follow-up information was obtained through clinic visit, telephone interview, written correspondence, medical records, and surveillance imaging reports. Late adverse event was defined as experiencing a repair failure or aortic valve structural deterioration with or without related reintervention. Repair failure was defined as failure directly involving the index repair (including any concomitant repair), namely pseudoaneurysm, fistula, endocarditis, or graft infection, and did not include subsequent repair necessitated by progression of aortic disease adjacent to the repair; some patients had more than one type of failure. Valvular-structural deterioration was defined as greater than mild aortic valve regurgitation or stenosis; both may occur simultaneously. The Social Security Death Index (up to 2011) and internet obituary searches were used to identify deaths among patients who were lost to follow-up.
Surgical techniques
We have described our techniques for ARR in detail elsewhere (16-21). Briefly, all patients underwent repair via median sternotomy using cardiopulmonary bypass. Patients underwent one of three basic approaches to ARR, and many types of ARR have been used over our lengthy experience. Although our approach to mechanical CVG has remained relatively constant over our larger surgical experience, the choice of tissue options has varied substantially; initially, patients were offered homografts to treat aneurysms when there was a need to avoid anticoagulation, shifting to bioprosthetic porcine roots, and later to VS approaches where possible. Additionally, we aimed to isolate the coronary arteries with minimal aortic tissue as part of a button reattachment strategy; in case of reoperation or other complicating factors, an alternate strategy of reattachment (e.g., Cabrol approach) was used. Poor quality leaflets may preclude the use of VS approaches, and a variety of techniques may be needed in these complex patients. When repairs extended into the aortic arch, a period of hypothermic circulatory arrest was used. We often used finer sutures when performing repairs in patients with Marfan syndrome. Importantly, in selecting the operative approach, we strived to facilitate subsequent aortic repair whenever possible, especially when aortic dissection is present.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 28 (IBM Corp., Armonk, NY, USA), and R version 4.2.2 from The R Project for Statistical Computing. Continuous data was tested for assumptions of normality using the Shapiro-Wilk test, determined to be abnormally distributed, and presented as median [Quartile 1–Quartile 3]. Categorical data was presented as count (percentage). Univariate comparisons across the three groups were conducted with the Pearson chi-square test, Fisher exact test, or nonparametric Kruskal-Wallis test, as appropriate. Late events were analyzed by the Kaplan-Meier and competing risk methods. A P value less than 0.05 was considered statistically significant.
Results
Preoperative characteristics
The overall median patient age was 38 [29–52] years. In comparing VS and VR groups, patients were similar in age and rates of major comorbidities and symptoms (Table 1). However, patients undergoing VS repair were less likely to have chronic proximal aortic dissection or a prior proximal aortic repair. Many patients undergoing mechanical CVG repair had a complex aortic history, with 1 in 5 experiencing a failure of a prior proximal aortic repair, and many patients have multiple prior aortic interventions at the time of ARR.
Table 1
| Variable | Mechanical CVG (n=113) | Valve sparing (n=62) | Bioprosthetic root (n=48) | P value |
|---|---|---|---|---|
| Age, years | 36 [30–47] | 38 [28–51] | 43 [33–57] | 0.3 |
| Male | 72 (64%) | 38 (61%) | 27 (56%) | 0.7 |
| Proximal aneurysm without dissection | 83 (73%) | 62 (100%) | 39 (81%) | <0.001 |
| Any aortic dissection | 37 (33%) | 10 (16%) | 17 (35%) | 0.046 |
| Aortic dissection (proximal aortal) | 30 (27%) | 0 | 9 (19%) | <0.001 |
| Chronic DeBakey type I | 25 (22%) | 0 | 9 (19%) | <0.001 |
| Prior DeBakey type II | 5 (4%) | 0 | 0 | 0.08 |
| Chronic DeBakey type III (distal aorta) | 7 (6%) | 10 (16%) | 8 (17%) | 0.08 |
| Aortic root diameter, mm | 55 [50–60] | 50 [47–53] | 52 [50–56] | <0.001 |
| Coronary artery disease | 12 (11%) | 4 (7%) | 9 (19%) | 0.1 |
| Cerebrovascular disease | 14 (12%) | 2 (3%) | 4 (8%) | 0.1 |
| Chronic kidney disease | 5 (4%) | 2 (3%) | 4 (8%) | 0.5 |
| COPD | 4 (4%) | 1 (2%) | 3 (6%) | 0.4 |
| Current tobacco use | 36 (32%) | 19 (31%) | 21 (44%) | 0.3 |
| Rupture | 0 | 0 | 0 | – |
| Symptomatic | 56 (40%) | 38 (61%) | 23 (48%) | 0.3 |
| Acute | 7 (6%) | 0 | 3 (6%) | 0.1 |
| Chronic | 48 (43%) | 37 (60%) | 20 (42%) | 0.07 |
| Peripheral vascular disease | 1 (1%) | 3 (5%) | 6 (13%) | 0.005 |
| Bicuspid aortic valve1 | 11 (10%) | 1 (2%) | 5 (10%) | 0.1 |
| Normal LV ejection fraction (≥55%) | 62 (55%) | 45 (73%) | 31 (65%) | 0.06 |
| Aortic valve regurgitation | ||||
| None | 17 (15%) | 23 (37%) | 11 (23%) | 0.004 |
| Mild | 26 (23%) | 18 (29%) | 8 (17%) | 0.3 |
| Moderate | 38 (34%) | 15 (24%) | 15 (31%) | 0.4 |
| Severe | 30 (27%) | 6 (10%) | 14 (29%) | 0.02 |
| Unknown | 2 (2%) | 0 | 0 | 0.4 |
| Aortic valve stenosis | ||||
| None | 106 (94%) | 62 (100%) | 47 (98%) | 0.09 |
| Mild | 0 | 0 | 0 | – |
| Moderate | 1 (1%) | 0 | 0 | 0.6 |
| Severe | 2 (2%) | 0 | 0 | 0.4 |
| Unknown | 4 (4%) | 0 | 1 (2%) | 0.3 |
| Prior proximal aortic repair | ||||
| Any prior proximal aortic repair | 38 (34%) | 0 | 9 (19%) | <0.001 |
| Root replacement | 13 (13%)2 | 0 | 4 (8%) | 0.02 |
| Ascending/arch repair/replacement (non-root) | 25 (24%) | 0 | 2 (4%) | <0.001 |
| With AV repair/replacement3 | 14 (14%) | 0 | 3 (6%) | 0.01 |
| Failure of prior aortic repair4 | 23 (22%) | 0 | 7 (15%) | <0.001 |
| Bioprosthetic AV regurgitation | 3 (3%) | 0 | 1 (2%) | 0.4 |
| Native AV regurgitation (prior resuspension) | 9 (8%) | – | 3 (6%) | 0.08 |
| Prosthetic aortic valve stenosis | 3 (3%) | 0 | 0 | 0.1 |
| Pseudoaneurysm | 8 (7%) | 0 | 4 (8%) | 0.047 |
| Infection/endocarditis | 1 (1%) | 0 | 2 (4%) | 0.1 |
Values are n (%) or median [Quartile 1–Quartile 3]. 1, congenital or functional bicuspid aortic valve present at the time of repair. 2, includes one prior David II valve-sparing aortic root replacement. 3, AV repair involved valve resuspension. 4, more than 1 type of failure is possible. CVG, composite valve graft; COPD, chronic obstructive pulmonary disease; LV, left ventricular; AV, aortic valve.
Operative details
The overall rate of redo sternotomy was 24% (n=54); however, this rate differed by type of repair, ranging from 2% to 37%. There was no difference in the duration of cardiopulmonary bypass (Table 2). Although the duration of aortic clamp and cardiac ischemic times statistically differed, these were not considered clinically significant, varying less than 15 minutes. Many patients underwent aortic arch repair—this ranged from 30% to 54% depending on the type of ARR.
Table 2
| Variable | Mechanical CVG (n=113) | Valve sparing (n=62) | Bioprosthetic root (n=48) | P value |
|---|---|---|---|---|
| Characteristics of repair | ||||
| 1st aortic intervention1 | 69 (61%) | 55 (89%) | 29 (60%) | <0.001 |
| 2nd aortic intervention | 27 (24%) | 6 (10%) | 13 (27%) | 0.04 |
| 3rd or greater aortic intervention | 17 (15%) | 1 (2%) | 6 (13%) | 0.002 |
| Repair before 2005 | 73 (65%) | 14 (23%) | 16 (33%) | <0.001 |
| Reoperation (redo sternotomy) | 42 (37%) | 1 (2%) | 11 (23%) | <0.001 |
| Perfusion and ischemia | ||||
| CPB time, min | 154 [132–186] | 164 [146–213] | 162 [139–193] | 0.5 |
| Hypothermic circulatory arrest | 39 (35%) | 22 (36%) | 26 (54%) | 0.052 |
| HCA time, min | 28 [20–42] | 19 [15–22] | 24 [17–32] | 0.02 |
| Aortic clamp time, min | 89 [80–111] | 112 [102–143] | 99 [78–124] | <0.001 |
| Cardiac ischemic time, min | 104 [83–126] | 120 [105–143] | 114 [94–140] | 0.01 |
| Reattachment technique | ||||
| Left coronary artery | ||||
| Button | 88 (78%) | 61 (98%) | 43 (90%) | <0.001 |
| Cabrol | 21 (19%) | 0 | 2 (4%) | <0.001 |
| Other | 2 (2%) | 0 | 1 (2%) | 0.6 |
| Right coronary artery | ||||
| Button | 90 (80%) | 58 (94%) | 45 (94%) | 0.009 |
| Cabrol | 15 (13%) | 0 | 2 (4%) | 0.004 |
| Other | 7 (6%) | 5 (8%) | 0 | 0.2 |
| Aortic arch management | ||||
| Any aortic arch | 34 (30%) | 24 (39%) | 26 (54%) | 0.02 |
| Hemiarch | 25 (22%) | 22 (36%) | 21 (44%) | 0.02 |
| Total arch | 9 (8%) | 2 (3%) | 5 (10%) | 0.3 |
| Other concomitant procedures | ||||
| CABG | 6 (5%) | 3 (5%) | 2 (4%) | 0.95 |
| Mitral valve repair/replace | 13 (12%) | 5 (8%) | 7 (15%) | 0.6 |
Values are n (%) or median [Quartile 1–Quartile 3]. 1, aortic intervention captured the sequence of open or endovascular procedures on any aortic segment. The mechanical CVG cohort includes three patients in which a prior mechanical valve was retained during aortic root replacement. The valve-sparing cohort was entirely composed of reimplantation procedures (n=62). The bioprosthetic root cohort included repair using a CVG with a tissue valve (n=6), a porcine bioroot (n=34), a root homograft (n=7), and partial root replacement using a tissue valve but leaving one native sinus intact (n=1). CVG, composite valve graft; CPB, cardiopulmonary bypass; HCA, hypothermic circulatory arrest; CABG, coronary artery bypass graft.
Early outcomes
Operative death was uncommon [4% overall (10/223)] and ranged from 2% to 8% depending on the type of repair (Table 3). Notably, death appeared increased for patients undergoing reoperation [5/47 (11%) compared to those without prior repair 5/176 (3%); P=0.04; Table 4]. Overall, stroke was rare [1/223 (<1%)], and persistent renal failure necessitating dialysis was uncommon [5/223 (2%)]. However, cardiac complications were relatively frequent, ranging from 37% to 46% by type of ARR.
Table 3
| Variable | Mechanical CVG (n=113) | Valve sparing (n=62) | Bioprosthetic root (n=48) | P value |
|---|---|---|---|---|
| Operative death | 5 (4%) | 1 (2%) | 4 (8%) | 0.2 |
| Persistent stroke | 1 (1%) | 0 | 0 | 0.6 |
| Persistent renal failure‡ | 2 (2%) | 1 (2%) | 2 (4%) | 0.6 |
| Bleeding requiring reoperation | 4 (4%) | 3 (5%) | 3 (6%) | 0.7 |
| Cardiac complications | 50 (44%) | 23 (37%) | 22 (46%) | 0.6 |
| Arrhythmia | 37 (33%) | 15 (24%) | 15 (31%) | 0.5 |
| Cardiac failure | 10 (9%) | 3 (5%) | 4 (8%) | 0.6 |
| Pericardial effusion requiring drainage | 10 (9%) | 3 (5%) | 2 (4%) | 0.4 |
| Respiratory failure | 22 (20%) | 6 (10%) | 10 (21%) | 0.2 |
| Necessitating tracheostomy | 8 (7%) | 0 | 3 (6%) | 0.1 |
| Survivor ICU LOS, days | 3 [2–5] | 2 [2–4] | 3 [2–5] | 0.3 |
| Survivor overall LOS, days | 10 [8–13] | 7 [6–10] | 8 [7–13] | <0.001 |
Values are n (%) or median [Quartile 1–Quartile 3]. ‡, present at the time of hospital discharge or early death. CVG, composite valve graft; ICU, intensive care unit; LOS, length of stay.
Table 4
| Case | Age, years | Sex | Redo proximal | Type of ARR | POD | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 57 | Male | No | Bioroot | 43 | Cardiac failure and heparin-induced thrombocytopenia leading to sepsis and MSOF |
| 2 | 52 | Female | Yes | Bioroot | 19 | Unknown |
| 3 | 34 | Male | Yes | CVG-M | 13 | Respiratory failure, pneumonia and sudden cardiac arrest |
| 4 | 43 | Male | No | Bioroot | 10 | Cardiopulmonary failure, MSOF, and sepsis |
| 5 | 39 | Female | No | Bioroot | 6 | Cardiac arrest leading to MSOF |
| 6 | 42 | Male | Yes | CVG-M | 5 | Intracranial hemorrhage and stroke |
| 7 | 50 | Female | Yes | CVG-M | 5 | Acute thrombosis of interposition graft to right coronary artery causing heart and respiratory failure leading to MSOF |
| 8 | 43 | Female | No | CVG-M | 3 | Liver and renal failure leading to MSOF |
| 9 | 38 | Male | Yes | CVG-M | 1 | Cardiac failure |
| 10 | 61 | Female | No | VS | 0 | Ventricular fibrillation |
Early deaths occurred prior to hospital discharge and included any transfer to another hospital or long-term acute care clinic. ARR, aortic root replacement; POD, postoperative day; MSOF, multisystem organ failure; CVG-M, mechanical composite valve graft; VS, valve sparing.
Late outcomes
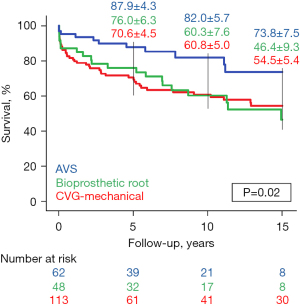
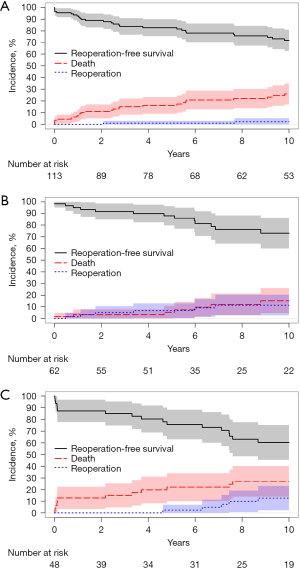
Of 213 early survivors, there were 7 (3%) patients who were lost to follow-up at the time of hospital discharge; these patients were presumed alive and censored at the time of discharge during analysis. All other early survivors [n=206 (97%)] had some measure of follow-up data available. Regarding late echocardiographic data, these were available in 138 early survivors (65%); however, patients undergoing VS repair or repair using a bioprosthetic root more commonly had available data [52/61 (85%) and 33/44 (75%), respectively]. The rates of late adverse events varied by type of ARR (Table 5), with patients receiving a mechanical CVG experiencing the fewest events. Survival differed by repair type (Figure 4) as did rates of reoperation (Figure 5). Survival was improved in patients who underwent VS repair, and post-hoc testing determined that these patients had higher rates of reoperation-free survival than did patients undergoing ARR using a bioprosthetic root (P=0.03).
Table 5
| Variable | Mechanical CVG (n=113) | Valve sparing (n=62) | Bioprosthetic root (n=48) | P value |
|---|---|---|---|---|
| Late adverse event | 5 (4%) | 11 (18%) | 10 (21%) | <0.001 |
| Repair failure | 5 (4%) | 7 (11%) | 8 (17%) | 0.03 |
| Involving root | 3 (3%) | 6 (10%) | 7 (15%) | 0.02 |
| Pseudoaneurysm | 2 (2%) | 1 (2%) | 2 (4%) | 0.6 |
| Infection/endocarditis | 2 (2%) | 2 (3%) | 0 | 0.5 |
| With reintervention | 3 (3%) | 7 (11%) | 8 (17%) | 0.006 |
| Valvular reintervention | 0 | 7 (11%) | 8 (17%) | 0.003 |
| Valvular-structural deterioration | 0 | 5 (8%) | 2 (4%) | 0.01 |
| Aortic valve regurgitation > mild | 0 | 5 (8%) | 2 (4%) | 0.01 |
| Aortic valve stenosis > mild | 0 | 1 (2%) | 1 (2%) | 0.3 |
| Subsequent repair | 23 (20%) | 2 (3%) | 5 (10%) | 0.005 |
| Of proximal aorta | 6 (5%) | 0 | 1 (2%) | 0.1 |
Values are n (%). Late adverse event was defined as experiencing a repair failure or aortic valve structural deterioration with or without related reintervention. Repair failure was defined as failure directly involving the index repair (which may have extended into the aortic arch), namely pseudoaneurysm, fistula, or endocarditis, graft infection, and did not include subsequent repair necessitated by progression of aortic disease adjacent to the repair; more than one type of failure was possible. Valvular-structural deterioration was defined as greater than mild aortic valve regurgitation or stenosis; both may occur simultaneously. Subsequent aortic repair was additional aortic repair unrelated to the aortic root that was performed to treat progressive aortic disease. CVG, composite valve graft.
Discussion
We found that repair in patients with MFS undergoing ARR resulted in generally low rates of operative mortality and stroke, despite many patients additionally undergoing aortic arch replacement. Elevated rates of aortic dissection, prior aortic intervention, reoperation, and concomitant repair demonstrate the unique challenges faced in this subset of complex patients. In general, we found that patients undergoing mechanical CVG repair tended to undergo a more complicated repair, with 37% of patients undergoing redo sternotomy.
Compared to the 316 patients described in the early AVOMP experience, (12) our subset of 223 patients had elevated rates of prior aortic surgery (especially prior ascending aortic replacement with aortic valve resuspension and prior ARR), aortic dissection, and concomitant aortic arch repair; in contrast, only 91% of AVOMP repairs were elective. Early outcomes were similar in our work and the AVOMP cohort, with low rates of operative death (4% vs. 1%, respectively) and stroke (1% vs. <1%, respectively), but substantial rates of cardiac complications (43% vs. 23%, respectively) (12). Regarding late findings, our results were similar to those of AVOMP in that patients undergoing VS repair tended to experience greater rates of valvular-structural deterioration, which did not appear to impact late survival (14). This finding is additionally supported by a meta-analysis of outcomes in 2,976 patients with MFS undergoing ARR by a CVG (n=1,624) or a VS (n=1,352) approach that found those undergoing VS had improved late survival (22). Other studies support this finding. The most recent study to describe the overall work of leading VS expert David (23) continues to demonstrate robust survival in patients with MFS undergoing ARR with VS reimplantation approaches, which was 95% at 10 years.
Importantly, technical aspects of VS repair centering on leaflet repair and coaptation parameters appear to drive durability (23-27). Another recent study summarizing the Washington University experience in a cohort of patients with and without MFS suggests that VS durability (freedom from reoperation and AR >2+) was improved when the coaptation effective height of the aortic valve leaflets following repair exceeded 10 mm (27). Our substantial rate of downstream subsequent aortic repair (ranging as high as 20% in patients undergoing mechanical CVG replacement) is explained by the progressive nature of aortic disease, especially in patients with chronic aortic dissection. The downstream aortic impact after proximal aortic replacement in patients with heritable thoracic aortic disease remains an issue of concern and in need of further evaluation. A study by Lenz and colleagues suggests that ARR itself may enhance rates of aortic dilatation (28). We highlight this finding to raise awareness that ARR is only one element of whole-patient care in those with MFS. In many such patients, progressive disease will necessitate additional repair of the distal aorta, and on occasion, replacement of the entire aorta. We believe such repair should center on open graft replacement (29), which we have demonstrated can be done with good results in most patients (16,18,30). Limitations of this study include the difficulty in evaluating a heterogeneous study population with a complicated history of aortic disease over a long period of time. Despite these limitations, we believe it is worthwhile to present our experience, including approaches to repair that are now less frequently used. Here, the complexity of aortic disease frequently necessitated a patient-tailored operative approach. Because of the long study period and the limits of our tertiary-practice service, we were unable to obtain follow-up echo data on all patients (most of whom are followed by a local clinician). Information that we were unable to systematically capture as part of late surveillance included late thrombolytic events, the late onset of aortic dissection, and late rupture events. Additionally, we may have failed to capture late deaths adequately, especially if deaths were unlikely to be recorded in the Social Security Death Index or online.
Conclusions
Aortic repair in patients with Marfan syndrome is complex and necessitates a patient-tailored approach with participation in a life-long surveillance protocol (16). We aimed to describe the scenarios of patient presentation for ARR and to underscore that patients selected for VS repair tend to differ from other patients in that they typically present with less complexity. Evaluating the type of ARR is difficult because of pervasive heterogeneity, the progression of aortic disease that necessities subsequent repair, and unclear late events (e.g., new onset dissection, late rupture, and late complications of multiple prior repairs) that undoubtedly affect long-term survival. What patients should know is that there are multiple approaches in the surgeon’s toolkit that can be drawn upon to fit a specific circumstance, including more than one option for a tissue-based repair.
Acknowledgments
The authors thank Scott A. Weldon, MA, CMI, FAMI, of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine, for creating several of the illustrations. Mr. Weldon’s work is partly supported by the E. Stanley Crawford Endowment. We thank Arin C. Jobe, MPH, Ginger M. Etheridge, BBA, Liza N. Hirsch, DBA, Veronica A. Glover, PhD, Katia Matar, BS, of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine, for providing project support. Dr. Coselli’s work is supported in part by the Cullen Foundation Endowed Chair at Baylor College of Medicine. Dr. LeMaire’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine. Dr. Moon’s work is supported in part by the Denton A. Cooley, MD Chair in Cardiac Surgery at the Texas Heart Institute and Baylor St. Luke’s Medical Center. Sincere thanks to James L. Dettore, Chief Executive Officer and Chairman of Brand Institute, for his generous support of our Aortic Surgery Patient Database.
Funding: None.
Footnote
Conflicts of Interest: SAL consults for Terumo Aortic and Cerus and has served as a principal investigator for clinical studies sponsored by Terumo Aortic and CytoSorbents. JSC serves as principal investigator, consults for, and receives royalties and a departmental educational grant from Terumo Aortic; consults and participates in clinical trials for Medtronic, Inc., and W.L. Gore & Associates; and participates in clinical trials for Abbott Laboratories, CytoSorbents, Edwards Lifesciences, and Artivion. MRM advises Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417-26. [Crossref] [PubMed]
- Murdoch JL, Walker BA, Halpern BL, et al. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804-8. [Crossref] [PubMed]
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Yacoub MH, Gehle P, Chandrasekaran V, et al. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080-90. [Crossref] [PubMed]
- Miller DC. Rationale and results of the Stanford modification of the David V reimplantation technique for valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2015;149:112-4. [Crossref] [PubMed]
- Miller DC. Valve-sparing aortic root replacement in patients with the Marfan syndrome. J Thorac Cardiovasc Surg 2003;125:773-8. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21.
- David TE, David CM, Manlhiot C, et al. Outcomes of Aortic Valve-Sparing Operations in Marfan Syndrome. J Am Coll Cardiol 2015;66:1445-53. [Crossref] [PubMed]
- Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med 1999;340:1307-13. [Crossref] [PubMed]
- Volguina IV, Miller DC, LeMaire SA, et al. Valve-sparing and valve-replacing techniques for aortic root replacement in patients with Marfan syndrome: Analysis of early outcome. J Thorac Cardiovasc Surg 2009;137:1124-32. [Crossref] [PubMed]
- Volguina IV, LeMaire SA, Palmero LC, et al. Intraoperative conversion after surgical failure: an overlooked complication of aortic root replacement in Marfan patients? Tex Heart Inst J 2011;38:684-6.
- Coselli JS, Volguina IV, LeMaire SA, et al. Early and 1-year outcomes of aortic root surgery in patients with Marfan syndrome: a prospective, multicenter, comparative study. J Thorac Cardiovasc Surg 2014;147:1758-66, 67 e1-4.
- Miller DC. Three-Year Outcomes of Aortic Root Surgery in Marfan Syndrome Patients (AVOMP): A Prospective, Multi-Center, Comparative Study. Presented at AATS Annual meeting, April 30, 2018.
- Coselli JS, Volguina IV, LeMaire SA, et al. Midterm outcomes of aortic root surgery in patients with Marfan syndrome: A prospective, multicenter, comparative study. J Thorac Cardiovasc Surg 2023;165:1790-9 e12. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732-8. [Crossref] [PubMed]
- LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg 2006;81:2063-78. [Crossref] [PubMed]
- Cekmecelioglu D, Coselli JS. Valve-sparing versus valve-replacing aortic root operations in patients with Marfan syndrome. Shanghai Chest 2019;4:23-9.
- Coselli JS, Green SY, Price MD, et al. Results of open surgical repair in patients with Marfan syndrome and distal aortic dissection. Ann Thorac Surg 2016;101:2193-201. [Crossref] [PubMed]
- Coselli JS, Weldon SA, Preventza O, et al. Valve-sparing versus composite root replacement procedures in patients with Marfan syndrome. Ann Cardiothorac Surg 2017;6:692-6. [Crossref] [PubMed]
- Crawford ES, Coselli JS. Marfan's syndrome: combined composite valve graft replacement of the aortic root and transaortic mitral valve replacement. Ann Thorac Surg 1988;45:296-302. [Crossref] [PubMed]
- Orozco-Sevilla V, Whitlock R, Preventza O, et al. Redo aortic root operations in patients with Marfan syndrome. Int J Angiol 2018;27:92-7. [Crossref] [PubMed]
- Flynn CD, Tian DH, Wilson-Smith A, et al. Systematic review and meta-analysis of surgical outcomes in Marfan patients undergoing aortic root surgery by composite-valve graft or valve sparing root replacement. Ann Cardiothorac Surg 2017;6:570-81. [Crossref] [PubMed]
- Elbatarny M, David TE, David CM, et al. Improved Outcomes of Reimplantation vs Remodeling in Marfan Syndrome: A Propensity-Matched Study. Ann Thorac Surg 2023;115:576-82. [Crossref] [PubMed]
- Svensson LG. Root Reimplantation With Leaflet Repair. Semin Thorac Cardiovasc Surg 2019;31:153-4. [Crossref] [PubMed]
- Zeeshan A, Idrees JJ, Johnston DR, et al. Durability of Aortic Valve Cusp Repair With and Without Annular Support. Ann Thorac Surg 2018;105:739-48. [Crossref] [PubMed]
- Yokawa K, Henmi S, Nakai H, et al. Mid-term outcomes of valve-sparing root reimplantation with leaflet repair. Eur J Cardiothorac Surg 2020;58:138-44. [Crossref] [PubMed]
- Kachroo P, Kelly MO, Bakir NH, et al. Impact of aortic valve effective height following valve-sparing root replacement on postoperative insufficiency and reoperation. J Thorac Cardiovasc Surg 2022;164:1672-80 e3. [Crossref] [PubMed]
- Lenz A, Warncke M, Wright F, et al. Longitudinal follow-up by MR angiography reveals progressive dilatation of the distal aorta after aortic root replacement in Marfan syndrome. Eur Radiol 2023; in press.
- Waterford SD, Moon MR. Stent grafting in Marfan syndrome? We are not convinced. J Thorac Cardiovasc Surg 2018;156:1773-5. [Crossref] [PubMed]
- Ghanta RK, Green SY, Price MD, et al. Midterm Survival and Quality of Life After Extent II Thoracoabdominal Aortic Repair in Marfan Syndrome. Ann Thorac Surg 2016;101:1402-9. [Crossref] [PubMed]






