Aortic root remodeling
Introduction
Root remodeling was proposed by Yacoub as a valve-preserving operation to treat patients with tricuspid aortic valves (TAVs), aortic regurgitation (AR), and root aneurysm (1). The goal was to normalize valve form and function by replacing all dilated aortic root tissue and restoring normal geometry. The early results were positive, but the technique did not always result in adequate valve competence (2). In particular, the late results of the original series were suboptimal, a relevant proportion of patients developed recurrent and relevant AR (3). The inferior stability has been related to the lack of annular stabilization in root remodeling (4).
Based on the better preservation of cusp motion, we started routinely employing root remodeling for root aneurysm in 1995 (5). Based on visual assessment of valve form, we observed that root aneurysm not infrequently coexisted with cusp prolapse (6). Concomitant correction of cusp by shortening, i.e., plication of the free cusp margin was not only feasible, but the early and mid-term results appeared equivalent to valves not requiring cusp repair (7). In addition, we realized that cusp prolapse could be induced or aggravated through reduction of intercommissural distance in root replacement (8). In order to assess cusp configuration more objectively, we started measuring effective height (eH) with a caliper intraoperatively (height difference between annular plane and free cusp margin) (9). Based on a study on normal aortic valves we considered an eH of 9 mm as normal, if a normal amount of cusp tissue was present (10). The amount of cusp tissue was determined by measuring geometric height (gH; stretched cusp from nadir to free margin) (Figure 1). We thus used gH to select repairable valves (gH ≥18 mm) and eH as intraoperative guide for cusp repair.
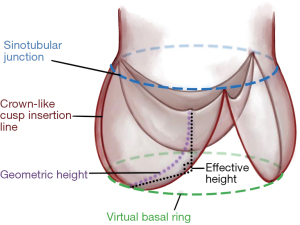
The positive results of a suture annuloplasty in bicuspid aortic valve repair prompted us to include it to root remodeling with a TAV (11). The early and late results showed improved aortic valve competence with the annuloplasty. In our hands, the technique of root remodeling has essentially remained constant with minor modifications. We have found the technique safe, reproducible, and easy to teach. It allows for standardized correction of root aneurysm with preservation of the vast majority of native TAV (12).
Operative techniques
Preparation
Preoperative assessment includes a baseline electrocardiogram, a chest X-ray, a cardiac computed tomography (CT) or coronary angiogram, a transthoracic echocardiogram, and a chest CT for redo-sternotomies.
A transesophageal echocardiogram (TEE) is performed in the operating room. Aortic dimensions such as the size of the aortic annulus, sinus of Valsalva, sinotubular junction (STJ) and ascending aorta are measured. To avoid projection artefacts, any two-dimensional long axis measurement is confirmed by short axis views. Three-dimensional TEE with multiplanar reconstruction yields even better information.
The cusps are assessed by multiplanar reconstruction, and gH and eH are measured for each cusp. Jet size and direction are carefully analyzed; an eccentric jet indicates a very high probability of cusp prolapse (>95%), but even in the presence of a central jet, some cusp prolapse will be found after correction of root dilatation in >45% (12).
Exposition
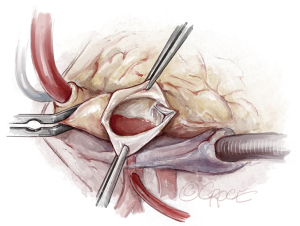
We use a median sternotomy with direct aortic cannulation in the proximal aortic arch and a two-stage venous cannula. On cardiopulmonary bypass, a vent catheter is placed in through the right superior pulmonary vein after cross-clamping the aorta. The ascending aorta is opened through a longitudinal incision to avoid cutting into a commissure (Figure 2). Cold blood cardioplegia is given via direct coronary ostial intubation and repeated every 20 to 30 minutes.
Operation
Exposure of the aortic root and aortic valve inspection
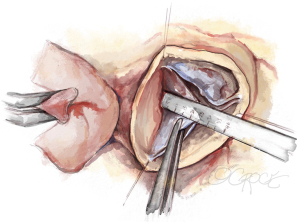
After the heart is arrested, the aorta is transsected 5 to 10 mm above the STJ. Stay sutures are placed above each commissure and fixed to the skin of the patient with clamps. The geometric height of each cusp is measured with a ruler (Figure 3) and the cusps are carefully assessed visually. If gH is at least 18 mm, the valve can be successfully repaired, unless other tissue abnormalities are encountered (large and multiple fenestrations, localized calcification). Smaller fenestrations are tolerated; they are repaired only if involved in the mechanism of obvious prolapse. Annular diameter is additionally determined by direct intubation.
Aortic root dissection
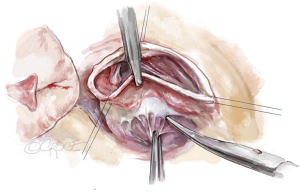
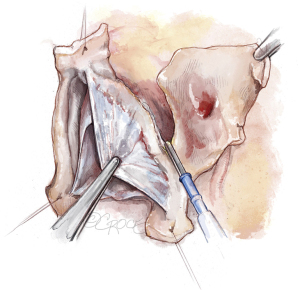
The aortic root is mobilized adequately; tension on the stay sutures facilitates exposure (Figure 4). This dissection does not have to be as deep as for the reimplantation technique. The sinuses are excised, leaving a rim of approximately 5 to 8 mm of aortic wall adjacent to the cusp insertion. The coronary orifices are mobilized. We usually excise the noncoronary sinus first. Most of the respective sinus wall is left on the coronary buttons (Figure 5).
Sizing and tailoring of the Dacron-graft
The size of the Dacron-graft is chosen according to the patient’s cusp size and body surface area (BSA). If gH is 20 mm and BSA is 1.8 to 2.2 m2, a graft size of 26 mm is appropriate; we use this in >90% of our patients. For patients with a BSA <1.8 m2, a graft size of 24 mm is chosen, and for patients with BSA >2.2 m2, a 28 mm graft will be adequate. If gH is <20 mm, graft size will be 2 mm less to create adequate configuration of the smaller valve.
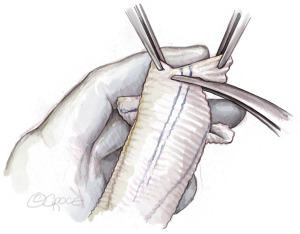
The graft is then tailored, creating three incisions at 120° angles. The incisions are initially 2 to 2.5 cm long on the stretched graft corresponding to approximately 1.5 cm in crimped stage. The incisions can later be extended if required. It is helpful to have a graft that already has marker lines with such a configuration (e.g., AlboGraft® Polyester Vascular Grafts, LeMaitre, Burlington, MA, USA). The tongues are cut in a rounded fashion (Figure 6).
Suturing of the graft
The graft is sutured to the cusp insertion lines using a 4–0 polypropylene suture, one suture for each sinus. We start in the nadir of the left cusp and suture both arms to the commissures. In order to avoid commissural height restriction, we vary the distance between the stitches on the graft. Close to the nadir, equal distance between the stitches (approximately 3 mm) is kept for graft and aortic wall remnant (Figure 7A). As we suture towards the commissure, the distance between stitches is increased to 5 to 6 mm on the graft, maintaining the 3 mm distance on the aorta (Figure 7B). This maneuver places more graft length into the sinus than the height of the native commissure and thus allows for unrestricted commissural height. In suturing, it is important that this suture line is hemostatic; tension must be kept on the suture at all times.
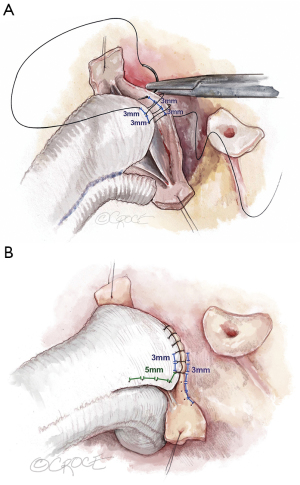
After completion of the left sinus, we suture the right, and finally the noncoronary, always following the identical principles. The sutures are tied at the commissures and kept long; they will subsequently be used as stay sutures for aortic valve assessment and repair.
Suture annuloplasty
In order to improve valve coaptation, we add a suture annuloplasty for an annular diameter >25 mm. Pulling the graft upwards will ensure the base of the repaired root is easily visible. A double-armed polytetrafluoroethylene (PTFE, Gore-Tex CV-0; WL Gore and Associates, Munich, Germany) suture is placed through septal tissue outside the left/right commissure. Posteriorly, the needle is passed through the bulging tissue at the nadir of the replaced left sinus (Figure 8A) and then through the tissue at the base of the noncoronary sinus. The anterior arm is passed through the tissue at the nadir of the right sinus and passed outside the right/noncoronary commissure (Figure 8B), avoiding interference with the membranous septum. It is then also passed through the tissue at the base of the noncoronary sinus.
The graft is shortened (usually 2 to 3 cm above the commissures with the graft stretched) and a Hegar dilator placed across the aortic valve. We choose the Hegar dilator according to BSA and gH of the cusp. For a gH of 20 mm, a 25 mm Hegar is chosen for a BSA >2 m2, 23 mm for smaller patients. If gH is less than 20 mm, the Hegar size will be reduced by 2 mm. The PTFE suture is then tied firmly around the Hegar dilator. A hemoclip placed on the PTFE suture helps to secure the knot (Figure 9).
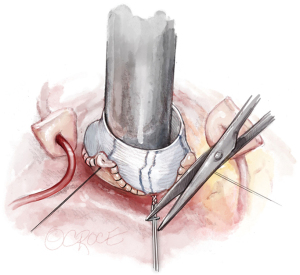
Valve assessment and repair
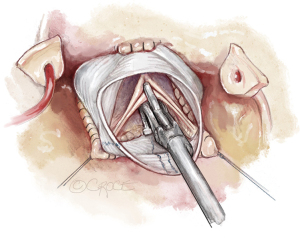
In assessing aortic valve form, one must consider the physiologic and pressurized conditions with the valve pushed downward and the commissures outward. In order to mimic this, the commissures are best pulled outward and upward using the long ends of the sinus sutures as stay sutures. Care must be taken to maintain the native commissural orientation and equal tension on the three sutures.
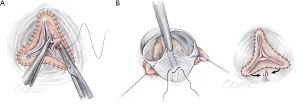
Visual inspection controls whether all free margins are at identical level. In addition, eH is measured on all three cusps with a caliper (Fehling Instruments, Karlstein, Germany) (Figure 10). Normal eH is 9 to 10 mm or 45% to 50% of gH. If the margins are not at identical level, the measurement helps in distinguishing between cusp restriction (eH too high) and prolapse (eH too low). Cusp prolapse can easily be corrected by central plication of the free margin (5-0 or 6-0 polypropylene) to shorten the margin (Figure 11A). This is done on all cusps until eH is 9 mm and the free margins are at identical height. Cusp restriction is corrected by reducing intercommissural distance of the respective cusp. This can easily be done by plicating the graft between the commissures of the respective cusp (Figure 11B).
Completion
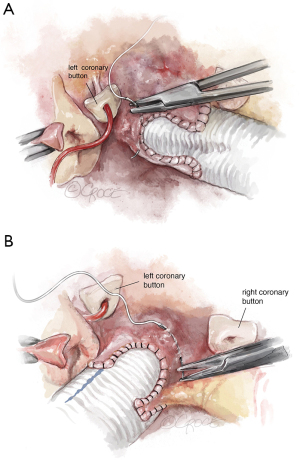
Once the aortic valve has adequate configuration, the coronary buttons are implanted into the graft (5-0 polypropylene). We usually begin with the right coronary ostium (Figure 12). Double bites of the redundant aortic button facilitate hemostasis. The left coronary ostium is implanted in identical fashion.
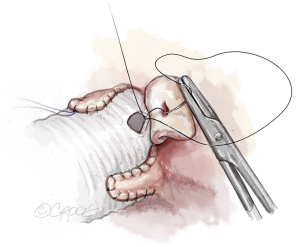
Graft length is estimated, avoiding excessive length. If the graft is long, it is shortened. If it does not reach the distal ascending aorta, a second segment of graft is added. The distal aortic anastomosis is performed with 4-0 polypropylene suture. The heart is de-aired and coronary circulation resumed. The aortic valve is now assessed by TEE. In the first few minutes of reperfusion any residual regurgitant jet will be larger than at the end of the procedure; this is the “hardest test phase” for echocardiographic control. If a residual leak (AR > grade I) is observed—particularly if eccentric—operative revision and repair of residual cusp pathology must be considered.
The patient is weaned from cardiopulmonary bypass and the aortic valve is reassessed by TEE. Central trivial or mild regurgitation is acceptable and in most instances related to the folds of cusp tissue after central cusp plication. At the time of discharge, the degree of AR is usually less than intraoperatively. Any eccentric jet with more than trivial severity indicates residual cusp pathology—i.e., restriction or prolapse—and be managed by reintervention.
Comments
After positive early results, root remodeling was found to yield suboptimal aortic valve durability, both in root aneurysm and acute dissection (13). In particular, this appeared to be the case in patients with connective tissue disease, even though 10-year freedom from reoperation was identical to reimplantation (14). Others have also found increased rates of reoperation, and most have related this to the lack of annular stabilization (14). Detailed analyses of the valve failures, however, have not been published.
Stimulated by the early results of Yacoub (3) and the better preservation of cusp motion (15), we started routinely employing root remodeling for root aneurysm in 1995 (5). We found that apparent cusp prolapse after completion of the root procedure could reproducibly be corrected by concomitant cusp repair. The tailoring of the cusps could be improved by intraoperative measurement of eH (9). As cusp retraction has been the most common non-repairable concomitant cusp pathology, the measurement of geometric cusp height facilitated selection of repairable cusps (10). Finally, the addition of an annuloplasty has improved early and late valve competence.
With these minor modifications, we have kept the technique essentially unchanged. Overall, we performed root remodeling in more than 710 instances of root aneurysm and TAV with an operative mortality of only 1.5% for elective procedures. We have found remodeling to be a relatively short procedure, saving more than 30 minutes of myocardial ischemic time compared to valve reimplantation. Our average myocardial ischemic time for isolated remodeling is currently 65 minutes, compared to approximately two hours for reimplantation in the hands of experienced surgeons and the senior author (13,16). In addition, remodeling is not limited in its applicability by muscle in the sinuses, i.e., discrepancy between aortoventricular junction and basal ring (17).
Root remodeling has become our preferred approach to all individuals with root enlargement and a non-calcified aortic valve irrespective of the degree of preoperative AR. Also, in acute dissection with pre-existent root dilatation, we have seen excellent valve stability with this operation (18).
Without annuloplasty, 10-year freedom from reoperation is 94%, with annuloplasty it is 97%. At 15 and 20 years, freedom from reoperation is 88% and 85% respectively, even without annular stabilization. Freedom from reoperation at 10 and 15 years is 96% with eH being measured, and 87% without eH being measured.
Root remodeling has been a reproducible form of valve-preserving root replacement. Measurement of gH facilitates the selection of repairable aortic valves. Adjusting cusp configuration according to intraoperative determination of eH has helped to expand the application of valve-preserving root replacement to a higher proportion of valves and has improved valve durability.
Acknowledgments
The authors would like to express their gratitude to Ms. Beth Croce for rendering and providing the professional surgical illustrations.
Funding: None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8.
- Luciani GB, Casali G, Tomezzoli A, et al. Recurrence of aortic insufficiency after aortic root remodeling with valve preservation. Ann Thorac Surg 1999;67:1849-52; discussion 1853-6. [Crossref] [PubMed]
- Yacoub MH, Gehle P, Chandrasekaran V, et al. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080-90. [Crossref] [PubMed]
- Erasmi AW, Sievers HH, Bechtel JF, et al. Remodeling or reimplantation for valve-sparing aortic root surgery? Ann Thorac Surg 2007;83:S752-6; discussion S785-90.
- Aicher D, Langer F, Lausberg H, et al. Aortic root remodeling: ten-year experience with 274 patients. J Thorac Cardiovasc Surg 2007;134:909-15. [Crossref] [PubMed]
- Langer F, Aicher D, Kissinger A, et al. Aortic valve repair using a differentiated surgical strategy. Circulation 2004;110:II67-73. [Crossref] [PubMed]
- Langer F, Graeter T, Nikoloudakis N, et al. Valve-preserving aortic replacement: does the additional repair of leaflet prolapse adversely affect the results? J Thorac Cardiovasc Surg 2001;122:270-7. [Crossref] [PubMed]
- Schäfers HJ, Aicher D, Langer F, et al. Preservation of the bicuspid aortic valve. Ann Thorac Surg 2007;83:S740-5; discussion S785-90. [Crossref] [PubMed]
- Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- Schäfers HJ, Schmied W, Marom G, et al. Cusp height in aortic valves. J Thorac Cardiovasc Surg 2013;146:269-74. [Crossref] [PubMed]
- Schäfers HJ, Aicher D. Root remodeling for aortic root dilatation. Ann Cardiothorac Surg 2013;2:113-6. [Crossref] [PubMed]
- Ehrlich T, Hagendorff A, Abeln K, et al. Aortic cusp abnormalities in patients with trileaflet aortic valve and root aneurysm. Heart 2022;109:55-62. [Crossref] [PubMed]
- David TE, Feindel CM, Webb GD, et al. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg 2006;132:347-54. [Crossref] [PubMed]
- David TE, Armstrong S, Maganti M, et al. Long-term results of aortic valve-sparing operations in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2009;138:859-64; discussion 863-4. [Crossref] [PubMed]
- Graeter TP, Fries R, Aicher D, et al. In-vitro comparison of aortic valve hemodynamics between aortic root remodeling and aortic valve reimplantation. J Heart Valve Dis 2006;15:329-35.
- Boodhwani M, de Kerchove L, Watremez C, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg 2011;141:917-25. [Crossref] [PubMed]
- Anderson RH. Clinical anatomy of the aortic root. Heart 2000;84:670-3. [Crossref] [PubMed]
- Kunihara T, Neumann N, Kriechbaum SD, et al. Long-Term Outcome of Aortic Root Remodeling for Patients With and Without Acute Aortic Dissection. Circ J 2017;81:1824-31. [Crossref] [PubMed]






