Cerebral protection in hemi-aortic arch surgery
Introduction Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
Surgical therapy for aortic arch disease usually requires a period of hypothermic circulatory arrest, which calls for cerebral protection strategies and adjuncts. The optimal strategy for protecting the brain from irreversible ischaemic damage during the period of circulatory arrest remains controversial. Patients present with diverse aortic pathologies and this may dictate different cerebral protection methods that are tailored for the circumstances of each individual case. The purpose of this overview is to describe each method of cerebral protection employed in hemi-aortic arch surgery and to explain their advantages and disadvantages. A surgical case on hemi-aortic arch replacement using retrograde cerebral perfusion is demonstrated (Video 1). We also present our hospital demographics and outcomes pertaining to cerebral protection in hemi-aortic arch surgery.
The current practices employed for brain protection during aortic arch surgery include: (I) deep hypothermic circulatory arrest (DHCA); (II) retrograde cerebral perfusion (RCP); and (III) selective antegrade cerebral perfusion (SACP).
Deep hypothermic circulatory arrest Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
DHCA has been in clinical practice for over 30 years and allows the surgeon to excise the distal clamp site, completely view the aortic anatomy in a bloodless field and perform a distal anastomosis without leaving any clamp-compromised tissue (1,2). The reasoning behind this technique is to reduce the brain’s activity and energy demand to a minimum.
There are two specific concerns about the use of DHCA:
- What temperature should be achieved before the extracorporeal circulation can be stopped?
- What is the anticipated ‘safe’ interval for a repair without neurological complications?
Most clinicians consider 35-40 mins of HCA at 20 °C as relatively safe, but there is increasing evidence that the interval could be a lot shorter. The most common complications of this approach are post-ischemic hypothermia, impaired autoregulatory mechanisms, and the abolishment of the brain protective barriers manifested by the increase in the cerebrovascular resistance that is initiated during the rewarming part of the procedure. To counteract these untoward effects, reperfusion and rewarming are established gradually and slowly. Additionally, the gradient temperature between the perfusate temperature and the core temperature should never exceed 10 °C. The metabolic management during this crucial phase also plays a pivotal part, supplemented with pharmacological adjunct such as Mannitol, which aids in the prevention of cerebral oedema and increased intracranial pressure, and also act as a free radical scavenger (3).
The advantages of DHCA include:
- A bloodless and motionless operative field;
- Avoidance of clamping and manipulation of the aorta with reduced risk for brain embolism;
- Simplicity and no need for additional perfusion equipment.
The disadvantages of DHCA include:
- Limited safe time of circulatory arrest;
- Prolonged cardiopulmonary bypass (CPB) time required to cool down and rewarm patients, which may result in an increased occurrence of pulmonary, renal, cardiac and endothelial dysfunction;
- Reperfusion injury;
- Clotting complications (4).
Svensson et al. (1) reported in a series of 616 patients an overall stroke rate and early mortality rate of 7% and 10%, respectively (median DHCA time: 31 mins; range, 7-120 mins). On univariate analysis, periods of circulatory arrest greater than 45 and 60 mins emerged as independent predictors of stroke and early mortality respectively. However, more recently, McCullogh et al. (5) demonstrated that the human cerebral metabolic rate is still 17% of baseline at 15 °C and that at this temperature the safe duration of circulatory arrest is no longer than 29 mins. Similarly, Reich et al. (2), Di Eusanio et al. (6) and Sakamoto et al. (7) have indicated that a duration of circulatory arrest of 25 mins is associated with an increased risk of transient neurological, memory and fine motor deficits.
For these reasons, the employment of DHCA seems to be rational only in patients requiring aortic arch repair with an anticipated duration of circulatory arrest shorter than 30 mins.
Retrograde cerebral perfusion Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
The use of RCP was originally reported by Mills and Ochsner (8) for the management of massive arterial air embolism during cardiopulmonary bypass in 1980. In 1982 Lemole and colleagues (9) described intermittent RCP as a method of facilitating intraluminal graft placement in the aorta. In 1990 Ueda and associates (10) first described the routine use of continuous RCP in thoracic aortic surgery for the purpose of cerebral protection during the period of obligatory interruption of anterograde cerebral flow.
There is compelling evidence that RCP may accomplish neuro-protection through providing cerebral metabolic support, expelling atheromatous and gaseous emboli from the cerebral vasculature, and maintaining cerebral hypothermia. The disadvantages in the use of RCP include cerebral oedema and the concern that very little of the perfusate actually reaches the brain to provide adequate neuroprotection.
The Safi group from Houston reported on the concept of an “opening” pressure that was required to observe a reversal flow in the middle cerebral arteries. 31 mmHg was required to open the venous capacitance vessels and overcome the jugular venous valves. This yielded acceptable results in terms of stroke (11).
The relationship between use of RCP and clinical outcome is also unclear. Some authors reported RCP duration to be a predictor of death and adverse neurological outcome (12-14), whereas others did not (15,16).
Current practice for RCP deployment is through a superior vena cava cannula with snaring of the caval cannula to prevent cardiac distention. The mode of application of RCP is uniformly accepted based on clinical observations, and anatomic and experimental data that support RCP with a pressurized entire venous system. Some centers limit the use of RCP to the prevention of neurologic injury in patients at high risk of embolic strokes. RCP could also be used in brief cycles to flush out the debris prior to the commencement of antegrade flow and reperfusion.
In summary, based on human and laboratory investigations, RCP neuro-protective mechanisms still remain controversial. When compared to SACP, RCP seems to be less effective whilst still providing some adjunctive brain protection compared to DHCA alone, due to continued cerebral cooling via the veno-arterial and veno-venous collateral circulations.
Selective antegrade cerebral perfusion Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
The first attempt to repair the aortic arch relied on complex methods of antegrade cerebral perfusion. In 1957, DeBakey reported a successful resection of an aortic arch aneurysm using normothermic CPB and cannulation of both subclavian and carotid arteries by means of several pumps (17). However, after early attempts, antegrade cerebral perfusion was abandoned due to unsatisfactory results and growing utilization of DHCA. SACP was then re-introduced by Frist et al. (18), Bachet et al. (19) and then popularized by Kazui et al. (20). They employed two separate pump heads for cerebral and systemic circulations and, in an elegant experimental study, indicated optimal cerebral flow rate (10 mL/kg/min) and perfusion pressure (40-70 mmHg) at 22 °C.
SACP provides several advantages: (I) the circulatory arrest time can safely be extended up to 90 minutes allowing more complex aortic repairs to be performed, (II) moderate (nasopharyngeal, 25 °C) instead of profound hypothermia can be used with reduced coagulative and systemic complications. Criticisms against SACP include technical complexity, reduced surgical visibility, and manipulation of the aortic arch and arch vessels especially in cases of acute dissections or severely atherosclerotic aortic arch aneurysms (21-23).
Although many experimental animal and patient cohort studies have been performed with SACP, only three prospective randomized controlled trials have compared SACP with RCP. Okita and associates (21) studied a total of 60 patients (30 with SACP and 30 with RCP) and found a decreased rate of total neurologic deficit in the SACP group (33% vs. 13%, P<0.05) but found no difference between groups in rates of death, stroke, or neurocognitive deficit. In an earlier report, Tanoue and colleagues (22) used transcranial Doppler ultrasonography to verify cerebral blood flow in 32 patients (15 with RCP and 17 with SACP). This study found improved cerebral blood flow in the SACP group. Only 3 patients in the RCP group showed evidence of reversal of cerebral blood. This low incidence of identification of flow reversal can be attributed to the technique of RCP used in the study: the superior vena cava pressure was only 15 to 25 mmHg. In addition, the cerebral perfusion time was 71 minutes in the SACP group, but only 38 minutes in the RCP group (P=0.0047). No differences in clinical outcomes were noted. Recent studies with SACP have reported excellent clinical outcomes, but variations in technique make it difficult to determine if SACP alone was responsible. The limitations, in common with most of such clinical studies, included differences in cannulation, delivery of perfusate (unilateral vs. bilateral), amount of perfusate and temperature of perfusate.
Neuromonitoring and avoidance of stroke Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
Neurologic complications following aortic surgery impose a negative impact and burden on patients’ quality of life. Several mechanisms are implicated, including cerebral embolism, cerebral hypoperfusion and inflammatory reactions. All of these mechanisms cause an imbalance between oxygen delivery and oxygen consumption in the brain. Neuromonitoring during aortic surgery may help to prevent injurious events or even detect them in a stage early enough to employ strategies to minimize secondary cerebral damage. While there are many modalities that can be used to demonstrate specific or regional brain oxygen deprivation during aortic surgery, all of these modalities have limitations.
Near infrared spectroscopy (NIRS) can be used to measure the cerebral tissue oxygen saturation of the bifrontal cortical regions. This method is non-invasive and works by emission of near infrared light and measurement of the absorption characteristics of oxy- and deoxyhemoglobin. Furthermore, transcranial Doppler (TCD) presents a non-invasive technique to monitor not only cerebral blood flow velocity, but also to detect cerebral emboli.
Finally, epi-aortic echocardiography is an important tool to help avoid or minimise cerebral injury during cardiac and aortic surgery. Even though this technique does not monitor the brain directly, it can be considered as a neuromonitoring technique in the broader sense.
Furthermore, the best maneuver to avoid stroke during complex aortic surgery is not only related to the sophisticated modality for neuromonitoring but also to the maneuvers that are employed when a regional drop in oxygen is detected. This includes checking the patient’s head position to ensure that it is not rotated, increasing the PaCO2 to above 40 mmHg, increasing the mean arterial pressure to above 60 mmHg, increasing the pump flow to 2.5 L/m2/min, raising the haematocrit above 20%, lowering the central venous pressure below 10 mmHg, increasing the inspiratory oxygen concentration, and deepening anaesthesia (24). In addition to this, scrupulous avoidance of manipulation of the diseased arch and cerebral vessels except during HCA is absolutely mandatory, as is careful, repeated aspiration of the cerebral vessels after circulatory arrest and before institution of antegrade flow (25,26).
The Liverpool Heart and Chest experience of hemi-aortic arch surgeryOther Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
Methods and results
We conducted a single-centre, retrospective study on a cohort of 125 consecutive patients who underwent aortic hemiarch replacement between June 1999 and September 2012 with either the antegrade or retrograde cerebral perfusion method. All clinical study data were collected prospectively during the patient’s admission and entered in a dedicated database as part of routine clinical practice. In-hospital outcomes were stratified by the method of cerebral protection.
Patient characteristics
All pre-operative patient characteristics are shown in Table 1. Risk factors were fairly well-balanced across the two groups. The median age of patients in the retrograde and antegrade groups were 63.3 (interquartile range=52.8, 72.3) and 64.4 (46.9, 71.1) years, respectively (P=0.65), the corresponding percentage of female patients in each group was 34.7% and 40.7% (P=0.56). The percentage of non-elective procedures in the retrograde group was half that of the antegrade group (9.2% vs. 18.5%), but due to small sample size this did not result in a statistically significant difference (P=0.18). Median Logistic EuroSCORE was also observed to be lower in the retrograde patients when compared to the antegrade (11.7 vs. 15.1) but this similarly did not result in a significant difference (P=0.58).
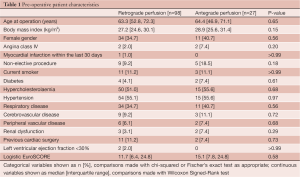
Full table
Procedural characteristics
There was a trend toward increased aortic crossclamp time in the RCP group compared to SACP (183 [IQR=149, 217] vs. 164 [119, 206] mins, P=0.06). No significant differences were observed between mean cardiopulmonary bypass time (P=0.16), circulatory arrest time (P=0.2) and nasopharyngeal temperature (P=0.13). These data are presented in Table 2.

Full table
Outcomes
Operative mortality occurred in 5 (5.1%) of the retrograde patients and 1 (3.7%) antegrade patient (P>0.99). Stroke occurred in 3 (3.1%) of the retrograde patients and 2 (7.4%) antegrade patient (P=0.29). Postoperative re-intubation occurred in 5 (5.1%) of the retrograde group compared to 4 patients (14.8%) in the antegrade group, and while this was a large difference, it did not reach statistical significance (P=0.1). ICU and postoperative length of stay were also comparable between the two groups and are shown along with the other postoperative complications in Table 3. Outcomes in the elective group only are also relatively equivalent and are shown in Table 4.
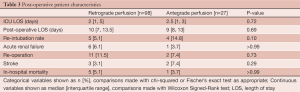
Full table
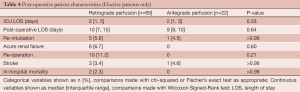
Full table
Conclusions Other Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
Due to the relatively short circulatory arrest time in hemi-aortic arch surgery, it is difficult to prove the superiority of one approach of cerebral protection over another. It is incumbent upon the surgeon to tailor each approach and strategy toward an individual patient with particular pathology in order to reduce mortality as well as cerebral complications. Because of the significant increase in adverse outcomes that are reported with DHCA, we do not advocate the use of this method alone for cerebral protection in hemi-aortic arch surgery.
AcknowledgementsOther Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Introduction
- Deep hypothermic circulatory arrest
- Retrograde cerebral perfusion
- Selective antegrade cerebral perfusion
- Neuromonitoring and avoidance of stroke
- The Liverpool Heart and Chest experience of hemi-aortic arch surgery
- Conclusions
- Acknowledgements
- References
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31.
- Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999;117:156-63.
- Hagl C, Khaladj N, Karck M, et al. Hypothermic circulatory arrest during ascending and aortic arch surgery: the theoretical impact of different cerebral perfusion techniques and other methods of cerebral protection. Eur J Cardiothorac Surg 2003;24:371-8.
- Di Bartolomeo R, Pilato E, Pacini D, et al. Cerebral protection during surgery of the aortic arch. MMCTS 2011;2010:004457.
- McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999;67:1895-9; discussion 1919-21.
- Di Eusanio M, Wesselink RM, Morshuis WJ, et al. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003;125:849-54.
- Sakamoto T, Zurakowski D, Duebener LF, et al. Combination of alpha-stat strategy and hemodilution exacerbates neurologic injury in a survival piglet model with deep hypothermic circulatory arrest. Ann Thorac Surg 2002;73:180-9; discussion 189-90.
- Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. J Thorac Cardiovasc Surg 1980;80:708-17.
- Lemole GM, Strong MD, Spagna PM, et al. Improved results for dissecting aneurysms. Intraluminal sutureless prosthesis. J Thorac Cardiovasc Surg 1982;83:249-55.
- Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553-8.
- Lee TY, Safi HJ, Estrera AL. Cerebral perfusion in aortic arch surgery: antegrade, retrograde, or both? Tex Heart Inst J 2011;38:674-7.
- Sasaguri S, Yamamoto S, Hosoda Y. What is the safe time limit for retrograde cerebral perfusion with hypothermic circulatory arrest in aortic surgery? J Cardiovasc Surg (Torino) 1996;37:441-4.
- Deeb GM, Williams DM, Quint LE, et al. Risk analysis for aortic surgery using hypothermic circulatory arrest with retrograde cerebral perfusion. Ann Thorac Surg 1999;67:1883-6; discussion 1891-4.
- Okita Y, Takamoto S, Ando M, et al. Mortality and cerebral outcome in patients who underwent aortic arch operations using deep hypothermic circulatory arrest with retrograde cerebral perfusion: no relation of early death, stroke, and delirium to the duration of circulatory arrest. J Thorac Cardiovasc Surg 1998;115:129-38.
- Wong CH, Bonser RS. Does retrograde cerebral perfusion affect risk factors for stroke and mortality after hypothermic circulatory arrest? Ann Thorac Surg 1999;67:1900-3; discussion 1919-21.
- Ueda Y, Okita Y, Aomi S, et al. Retrograde cerebral perfusion for aortic arch surgery: analysis of risk factors. Ann Thorac Surg 1999;67:1879-82; discussion 1891-4.
- DeBakey M, Crawford ES, Cooley DA, et al. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg Gynecol Obstet 1957;105:657-64.
- Frist WH, Baldwin JC, Starnes VA, et al. A reconsideration of cerebral perfusion in aortic arch replacement. Ann Thorac Surg 1986;42:273-81.
- Bachet J, Guilmet D, Goudot B, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102:85-93; discussion 93-4.
- Kazui T, Inoue N, Yamada O, et al. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53:109-14.
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9.
- Tanoue Y, Tominaga R, Ochiai Y, et al. Comparative study of retrograde and selective cerebral perfusion with transcranial Doppler. Ann Thorac Surg 1999;67:672-5.
- Di Eusanio M, Wesselink RM, Morshuis WJ, et al. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003;125:849-54.
- Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg 2007;104:51-8.
- Griepp RB. Cerebral protection during aortic arch surgery. J Thorac Cardiovasc Surg 2001;121:425-7.
- Hagl C, Ergin MA, Galla JD, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 2001;121:1107-21.





