Echocardiography of the aortic root: a practical approach for aortic valve-sparing surgery
Introduction
Since its first description in 1992 by David and Feindel (1), aortic valve repair (AVP) has been increasingly used for the treatment of both aortic aneurysm and aortic regurgitation (AR) and is now considered as a Class 2b indication in the current American and European guidelines (2,3). To date, AVP is being proposed for young and middle-aged patients with aortic valve (AV) dysfunction and/or aortic root dilatation, for those with a congenital AV malformation (most commonly bicuspid valve and, less often, unicuspid variants), and for those with a normal trileaflet AV configuration as well. In the various centers with adequate expertise that perform AVP, a standardized approach to AVP addressing both the aorta and the valve, combined with physiological reconstruction of the root (resuspension of the leaflets and annuloplasty of the aortic annulus), leads to excellent mid- and long-term results (4-8). Furthermore, AVP avoids the long-term risks of anticoagulation, allows growth, and postpones AV replacement to later in life. The incidence of valve-related complications is low, leaving young patients without any functional limitations (9). Parallel to the increasing use of this surgical technique, the understanding of the anatomical and functional mechanisms of AR and aneurysmal dilatation of the aortic root has also improved (10-13). Functionally, the aortic annulus, cusps, and sino-tubular junction (STJ) contribute to the valvular mechanism. All of them need to be evaluated when assessing the mechanism of AR, and especially when a surgical repair is being considered. For surgical correction to be effective, both cusp and root abnormalities must be evaluated and corrected. Indeed, the ultimate goal of reconstructive valve surgery is to restore a normal AV function and a normal aortic root shape as well (8). AV correction must be carefully planned. Thus, a thorough understanding of the mechanisms of AR and an appreciation of the anatomical architecture of the root are fundamental for successful and efficient repair. Accordingly, both transthoracic and transesophageal echocardiography (TEE) have gained substantial importance in the evaluation of AV diseases (10,14,15). Echocardiography combines several interesting technical aspects that allow an accurate assessment of the AV and root, including good spatial and temporal resolution and real-time assessment of valve function, which other imaging techniques are unable to provide (16). Technical advances have positioned TEE, and more specifically 3D TEE, as an indispensable tool and the cornerstone for operative decision-making, guidance of reconstructive techniques, and assessment of AVP adequacy (12,17). Real-time 3D imaging, by evaluating and quantifying the spatial and functional relationship among AV components, along with multiplanar reconstruction (MPR) that adjusts the orthogonal imaging planes for optimal visualization of coaptation lines and anatomical structures have become a clinical reality. In many patients considered for AVP, an imaging approach using exclusively echocardiography may be adequate, specifically when 3D echocardiography is feasible. In this review, we propose a practical approach to evaluate AV morphology and function as well as aortic root anatomy by using 3D TEE, with the aim of gaining accurate and relevant information for patients with AR and/or aortic root aneurysm in whom AVP is being considered. Additionally, we provide a practical evaluation approach to assess the immediate results obtained in the operating room, with a specific focus on what is an “acceptable AVP”.
Fundamental anatomy of the AV and aortic root
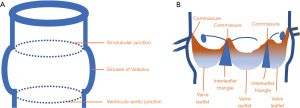
The aortic root is defined as the portion of the aorta that supports the AV leaflets, delineated cranially by the STJ and caudally by the ventriculo-aortic junction (VAJ) (12,13). It comprises the sinuses, leaflets, commissures, and interleaflet triangles (Figure 1). The STJ is circular and supports the peripheral attachments of the valve leaflets. The leaflets are inserted into the aortic wall in a semilunar fashion, and there is a commissure between two leaflets. A normal commissure almost reaches the level of the STJ. The leaflet closure determines the valve sealing in the central coaptation area. The level of the coaptation is at the middle distance between the nadir of their insertion and the commissural areas (18-20). The VAJ is defined by the nadirs of attachments of these leaflets and the interleaflet triangles bounded by the semilunar attachments of the leaflets. The term “aortic annulus” is commonly used to describe this area but is inaccurate, because it implies a circular structure while it actually corresponds to the combination of the crown-shaped cusp insertion lines. The aortic root functionally acts as an individual hemodynamic entity (20). The integrity of all the components of this entity is essential for a normal function, but besides the leaflets themselves, the borders of the aortic root (namely the STJ and the VAJ) are of paramount importance to ensure the proper functioning of the valve. AR occurs when one or both borders are dilated. The STJ and VAJ, because of their interdependence, are referred to as the functional aortic annulus (FAA).
Preoperative echocardiographic assessment of the AV and root
Understanding the interaction between the leaflets, annulus, commissural structures, and STJ allows the surgeon to propose a tailored AR repair, whether aortic root disease is present or not (21-23). TEE is particularly well adapted to identify lesions that are suitable for repair based on valve and root anatomy and on analysis of the AR mechanisms. The main advantage of echocardiography is that it allows the assessment of the AV and aortic root in real time and in a physiological hemodynamic state. By contrast, the surgeon can only assess the aortic root geometry and valve function in an “unpressurized” state (12). Therefore, it is preferable to evaluate the AV before general anesthesia and before placing the patient on cardiopulmonary bypass so as to obtain accurate measurements of the dimensions. Importantly, the functional anatomy of AR defined by TEE is strongly and independently predictive of valve reparability and postoperative outcomes, which underlines the crucial role of TEE before surgery and in the operating room (24,25). Valvular function should be documented by multiple 2D incidences and orthogonal views, and by live 3D ‘en face’ views. Current TEE transducers provide high frequencies and bandwidth, with increased resolution in 2D and live 3D as well. Furthermore, the main manufacturers of cardiac ultrasound machines currently propose 3D analysis software embedded in the machines. Standardized approaches and techniques enhance the reproducibility of AVP (8). Accordingly, based on our experience, we propose a systematic echocardiographic approach in six steps (Table 1). Essentially, our approach is based on 3D TEE imaging, particularly 3D MPR of the functional anatomy of the AV and aortic root, so as to facilitate the discussion between the imaging specialist and the surgeon and ultimately to plan an appropriate AVP. Based on this systematic analysis, typical features amenable to AVP are proposed in Table 2.
Table 1
| Steps | Observation(s) |
|---|---|
| 1. Determining the valve phenotype (Figure 2) | Type: |
| Tricuspid | |
| Bicuspid | |
| Fused BAV | |
| 2-sinus BAV | |
| Partial-fusion BAV | |
| Unicuspid | |
| Quadricuspid | |
| Commissural height | |
| 2. Determining the root phenotype (Figure 3) | Type: |
| Normal phenotype | |
| Ascending phenotype | |
| Root phenotype | |
| Extended phenotype | |
| Annulus diameter | |
| Valsalva sinus diameter | |
| STJ diameter | |
| Tubular ascending aorta diameter | |
| 3. Assessment of aortic regurgitation (Figures 4,5) | Quantification: |
| EROA | |
| Regurgitant volume | |
| Vena contracta width | |
| Vena contracta area | |
| Mechanism: functional classification based on leaflet motion and FAA dilatation: | |
| Type I a – b – c – d | |
| Type II | |
| Type III | |
| 4. Assessment of leaflet tissue quality (Figure 6) | Good quality: |
| Thin leaflet which keeps a curved aspect during the systole and a bending during the diastole | |
| Systolic “fluttering” or “trill” of the leaflet | |
| Poor quality: | |
| Thickening | |
| Calcification grading | |
| 1: no calcification | |
| 2: isolated small calcification spots | |
| 3: bigger calcification spots interfering with cusp motion | |
| 4: extensive calcifications of all cusps with restricted cusp motion | |
| 5. Specific measurements of leaflet geometry (Figure 7) | Commissural height |
| Commissural orientation in case of BAV | |
| Coaptation height | |
| Effective height | |
| Geometric height | |
| Free margin length | |
| 6. Looking beyond the valve (Figure 8) | Localization of the coronary arteries |
| Assessment of the pulmonary valve |
BAV, bicuspid aortic valve; STJ, sino-tubular junction; EROA, effective regurgitant orifice area; FAA, functional aortic annulus.
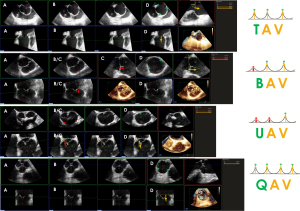
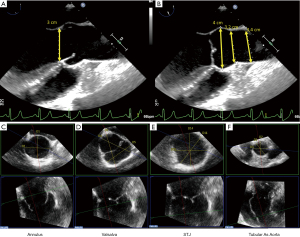
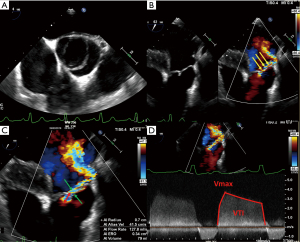
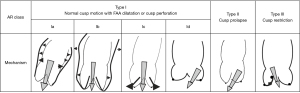
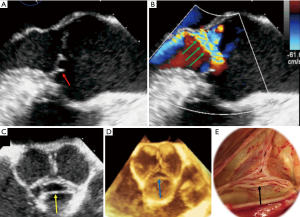
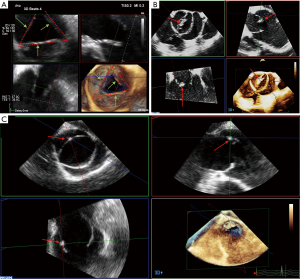
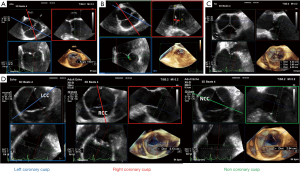
Table 2
| Consider repair | Discussion with balancing of repair and replacement | Consider replacement | |
|---|---|---|---|
| Valve phenotype | BAV: Type A and B | UAV | |
| TAV | BAV: Type C | ||
| QAV | |||
| Root phenotype | All phenotypes | ||
| AR severity | All grades | ||
| Mechanism of AR | Type Ia–d | Type Id with large/multiple perforations | Type Id due to active endocarditis |
| Type II | Type II due to large/multiple fenestrations | ||
| Type III in young/congenital heart | Type III | ||
| Quality of cusp tissue | Flexible cusps, no SRCM | SRCM due to body calcifications (Grade 2) | Major tissue thickening |
| SRCM due to root dilatation | Grade 3–4 calcifications | ||
| SRCM due to free margin thickening | Active and destructive endocarditis | ||
| Grade 1–2 calcifications | |||
| Specific measurements | TAV: gH >16 mm | TAV: gH ≤16 mm | |
| BAV: gH >19 mm (FC) | BAV: gH ≤19 mm (FC) | ||
| Pre- and post-repair eH, cH, and CO must be compared | |||
BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; QAV, quadricuspid aortic valve; UAV, unicuspid aortic valve; AR, aortic regurgitation; SRCM, systolic restrictive cusp motion; FC, fused cusp; gH, geometric height; eH, effective height; cH, coaptation height; CO, commissural orientation.
Determining the valve phenotype
It is important to identify the exact valve phenotype among the many existing, because of its impact on the surgical decision. The most common type is the classical tricuspid aortic valve (TAV). Bicuspid aortic valve (BAV) is the second most frequently encountered type. Despite its low prevalence (<1%), the latter is frequently observed in cardiac patients, because of its usual association with aortic stenosis, AR, and aortopathy (27,28). According to a recent international consensus, from the valvular perspective, BAV has three major phenotypic expressions: (I) the fused BAV that accounts for more than 90% of the cases, (II) the 2-sinus BAV, and (III) the partial-fusion (forme fruste) BAV. From the aortopathic perspective, BAV has three major phenotypic expressions: (I) the ascending phenotype that represents more than 70% of the cases, (II) the root phenotype (20% of cases), and (III) the extended phenotypes (28). Unicuspid aortic valve (UAV) and quadricuspid aortic valve (QAV) are even rarer, but again, they are often associated with valvular stenosis or leak (29). In some cases, determining the valve type can be challenging. This is particularly true when the sonographer must distinguish between a very asymmetric BAV on the one hand and a UAV or a classical TAV on the other hand in the presence of commissural and/or raphe calcifications. A UAV can actually be reconstructed as a symmetric BAV, and a very asymmetric BAV as a true TAV. The general principle is that the number of “functional” commissures determines the phenotype. A TAV has typically three functional commissures at the same height. A BAV has two functional commissures (sometimes with an asymmetric height) and a non-functional commissure with a hypoplastic interleaflet triangle: the raphe. A UAV has two non-functional commissures and a single functional commissure, while a QAV has four commissures, one of which is in most cases underdeveloped (8,30). The height of the commissures is assessed using MPR based on a 3D volume acquisition of the FAA. The first step is to reconstruct an orthogonal short-axis view (“en face view”) of the AV in diastole (Figure 2). Then, the short-axis plane is gradually displaced from the coaptation plane cranially to the top of the leaflets in order to identify the (functional or non-functional) commissural insertions. The commissural height (CH) is the distance between the aortic annulus, which corresponds to the basal attachment of the leaflet within the left ventricle, and the edge of the commissure.
Determining the root phenotype
Again, the term “aortic root” refers only to the most proximal part of the ascending aorta, from the annulus to the STJ, formed by the sinuses of Valsalva and containing the AV. There are four different phenotypes requiring different surgical repair strategies: a normal aorta, an ascending phenotype with preferential dilatation of the tubular ascending aorta, the root phenotype with preferential dilatation of the root, and the extended phenotype showing dilatation of the root, ascending aorta, and arch (30). Aortic dilatation is not synonymous with regurgitation because of elongation of the free margin and increase in AV leaflet size (16). The measurements of the aortic root and ascending aorta are generally performed on a mid-esophageal 2D long-axis view of the AV and ascending aorta, at 110–130° (Figure 3). Since the annulus diameter is the largest in systole, it should be measured in mid-systole, while the Valsalva sinuses, STJ, and ascending aorta should be measured in end-diastole. The spatial resolution of modern 3D TEE allows the sonographer to use the inner edge-to-inner edge method, like in magnetic resonance and computed tomography (26,31). Modern ultrasound scanners provide additional values thanks to MPR to guide the surgeon. The orthogonal triple alignment avoids an under- or overestimation of these measurements because of the oblique orientation of the AV and FAA (17). Since the annulus is often elliptic in TAV and BAV patients, with variable diameters, it is preferable to measure the annulus in a cross-sectional view, using 3D imaging (32). The ellipticity index of the annulus corresponds to the ratio between the smallest and the tallest diameter. A value >1.10 is considered as elliptic (17). The cut-off value of 25 mm guides the surgical decision to stabilize the annulus with different options, such as suture annuloplasty, external ring, or valve-sparing reimplantation (33,34). Similarly, the Valsalva sinuses are measured on three different axes in order to detect a sinus asymmetry that can help the surgeon to choose between the remodeling technique or the reimplantation procedure. The STJ is an important part of the FAA, theoretically delimited by the edge of the commissures. In case of ascending aorta dilatation, this structure is sometimes difficult to identify on a 2D long-axis view because the sonographer cannot localize it precisely. Moving and rotating the short-axis plane on the MPR helps to find and characterize the true dimensions of the STJ. The STJ diameter is useful to determine the choice of the aortic graft size. An undersizing may cause a cusp prolapse and iatrogenic residual AR. Finally, the tubular portion of the ascending aorta must be measured by MPR to find the larger true diameter.
Assessment of AR
Assessing the mechanism and quantifying the AR have important implications regarding the indication for an intervention and the type of surgery. There are several methods to evaluate AR severity. As none of them is 100% accurate, AR severity should be assessed using all the information collected during the examination in an integrative and comprehensive way (10,31). The criteria for grading AR (35) are summarized in Table 3. The values derived from the proximal isovelocity surface area (PISA), such as the effective regurgitant orifice area (EROA) and the regurgitant volume (RV) (Figure 4), are highly relevant as they are independent predictors of clinical outcome, especially in asymptomatic patients (36). Of note, the loading conditions under general anesthesia are, in most cases, lower than for an awake patient. Therefore, these quantitative parameters can be underestimated and should be assessed preoperatively in normal loading conditions. The vena contracta (VC) width is a semi-quantitative parameter that can more easily be assessed in mid-esophageal long-axis view and it seems less dependent of the loading conditions. The VC represents the smallest flow diameter at the level of the AV in the left ventricular outflow tract (LVOT), immediately below the flow convergence region. For the VC width measurement to be reliable, the regurgitant orifice must have a circular shape. Unfortunately, in many instances (and particularly in BAV patients), the orifice is elliptic or irregular. To overcome this problem, 3D color Doppler echocardiography has been shown to be a useful tool for visualizing the actual shape of the regurgitant orifice and it can be used to measure the VC area (10). The VC area is the orthogonal reconstruction of the VC and it corresponds to the EROA, obtained with a color MPR. It is more representative than the VC alone. Usually, the transvalvular gradients and the Doppler color flow are obtained in a deep trans-gastric view. It can be difficult to align the Doppler beam to evaluate the regurgitant flow in the presence of a leaflet prolapse. A very common situation is a right coronary leaflet prolapse, with an eccentric jet toward the anterior mitral leaflet. In this particular situation, the regurgitant flow can be assessed in a mid-esophageal long-axis view, at 120°. There are two main mechanisms for AR: FAA dilatation and pathological leaflet. The functional classification described by our group distinguishes three different mechanisms based on the leaflet function and considering the aortic root as a functional entity: the FAA (Figure 5) (18,37). Importantly, this proposed classification system encompasses all AR types, provides a common language for communication between the different disciplines (imaging specialists and surgeons), guides the repair techniques used, and can help to predict mid-term outcome. This classification is centered around the idea that the AV, in a very similar way to the mitral valve, consists of two major components, the aortic annulus and the valve leaflets. Unlike that of the mitral valve, however, the annulus of the AV is not a single anatomical structure. The FAA rather consists of two separate components, the VAJ, or aortic annulus, and the STJ, as explained previously. As in the Carpentier classification of mitral valve disease, three major AR mechanisms can be identified (37):
- Regurgitation associated with normal leaflet motion is referred to as type I. Type I AR is largely due to lesions of the functional aortic annulus causing a central jet, with type Ia AR resulting from STJ enlargement, type Ib resulting from Valsalva sinus and STJ dilatation (extended phenotype) leading to leaflet tethering by displacement of the commissures, type Ic resulting from VAJ dilatation, and type Id resulting from cusp perforation without a primary FAA lesion.
- Type II AR is due to excessive leaflet motion as a result of excessive leaflet tissue or commissural disruption causing an eccentric jet. Different excessive leaflet motion can be described. First, a billowing occurs when the body of the leaflet passes under the annular plane while the coaptation is still above the annular plane. Despite a loss of coaptation, billowing is not necessarily associated with a significant regurgitation. Second, the cusp prolapse is defined by a coaptation overriding the annular plane, typically producing an eccentric jet. A fibrous band (mostly identified by the surgeon) often coexists with a prolapse. On echocardiography, the prolapse can exhibit two different aspects. It can look like a transverse fold of the cusp, producing a partial and relative cusp prolapse, as it can be visualized in the long-axis view at 120–130° (Figure 6). It can also affect the whole leaflet, producing the “spoon sign” (a circle on the middle of the leaflet) in the short-axis view, at 45°. Leaflet fenestrations are a congenital variance comprising a gap with no leaflet tissues in a small area of the leaflet, near the commissure and just below the free margin (8). In certain cases, fenestration is involved in the prolapse mechanism (i.e., by elongation or rupture of the free margin in relation to the fenestration) but is hardly identified by the imaging specialist. Finally, a flail is described when the aortic side of the cusp passes into the LVOT, corresponding to a complete eversion of the cusp.
- Type III AR generates either a central or an eccentric jet and is due to leaflet restriction, which may be found in degenerative or rheumatic valvular disease as a result of calcification, thickening, and fibrosis (poor quality and quantity) of the AV leaflets. Type III AR must generally be considered as non-repairable due to the risk of recurrent post-repair AR (24,33).
Table 3
| Qualitative | Mild | Moderate | Severe |
|---|---|---|---|
| Valve morphology | – | – | Flail |
| Large coaptation defect | |||
| Color flow regurgitant jet area | – | – | Large in central jets |
| Variable in eccentric jets | |||
| Semiquantitative | |||
| VC width (mm) | <3 | 3–6 | >6 |
| VC area (mm2) | <30 | 30–50 | >50 |
| Quantitative | |||
| EROA (mm2) | <10 | 10–30 | >30 |
| RV (mL/beat) | <30 | 30–60 | >60 |
VC, vena contracta; EROA, effective regurgitant orifice area; RV, regurgitant volume.
It should be noted that several AR types can be intertwined. For example, a cusp prolapse in a dilated root must be described as a combination of type Ib and type II. Most type I and II ARs are eligible for a conservative surgery.
Quality of cusp tissue
Leaflet tissue quality is an important feature for surgeons so that they can anticipate the repair strategy. However, it must be acknowledged that the qualitative assessment of leaflet tissue is a big challenge for the sonographer. Although there are no real objective criteria to define which tissue is repairable or not, the sonographer can identify some clues and markers in favor of reparability. Good leaflet mobility is defined by a thin leaflet that keeps a curved aspect during the systole and a bending during the diastole. A systolic “fluttering” or “trill” of the leaflet appears in case of equalization of pressures between the left ventricle and the aorta and it can also be considered has a good sign of flexibility. The absence of restrictive systolic motion or functional leaflet restriction (the stretched systolic aspect of the leaflet in short-axis view) is a sign of good tissue quality (Figure 7A). A systolic restrictive cusp motion (SRCM) is frequently seen in case of STJ dilatation. A thickened aspect of the free margins can alter the opening of the valve and generate an AR by decreasing the coaptation length (Figure 7B), but it does not represent an absolute contraindication for AVP. It must be identified by the imaging specialist, because a shaving of the free margins can restore more flexibility and function to the leaflets. Finally, it is crucial to identify and quantify leaflet calcifications in anticipation of surgery. Even a minor calcification (Figure 7C) in a strategic structure such as the commissure, along the free margin, or in the body of the cusp can severely impair the valvular function. Aortic calcification grading (1: no calcification, 2: isolated small calcification spots, 3: bigger calcification spots interfering with cusp motion, 4: extensive calcifications of all cusps with restricted cusp motion) must be taken into account when determining the surgical strategy and predicting AVP durability. Grades 3 and 4 are considered to be non-repairable (15). In presence of calcifications, an aortic stenosis must obviously be excluded, since it represents a contraindication for AVP. For this purpose, the continuity equation and/or the planimetry method can be used, the latter being less dependent of the loading conditions.
Specific measurements of leaflet geometry
Besides morphological evaluation, specific functional measurements related to the cusp geometry are mandatory for successful AVP. These include the CH, the commissural orientation (CO) in case of BAV, the coaptation height (cH), the effective height (eH), the geometric height (gH), and the free margin length (FML) (Figure 8). The method to measure the CH has been described above. In case of BAV, the measurement of the non-functional CH and the length of the cusp fusion of the fused cusp are intimately related to the CO. The CO of a BAV is an important factor to consider before AVP. The CO is defined as the angle formed by the lines joining the commissures to the central axis of the valve. The angle measured in diastole is that on the non-fused cusp side (8,34). Recently, a CO-based classification has been proposed (34).
- Type A has a CO between 160° and 180° (symmetric).
- Type B has a CO between 140° and 159° (asymmetric).
- Type C has a CO between 120° and 139° (very asymmetric).
The optimal scenario for AVP is the symmetric one. The CO influences the surgical approach and the long-term durability of the repair. Indeed, the goal of surgery is to “symmetrize” the valve and obtain a CO at 180°. A symmetrical CO is associated with better durability and flow characteristics across the aortic root. Regarding the CO, the sonographer must keep in mind three important points. First, a type A is easier to repair than a type C (which should probably be treated more like a TAV). Second, there is a positive correlation between the CO and the fusion length, and a negative correlation between the CO and the height of the non-functional commissure. Third, type A presents more frequently with isolated root dilatation, while type B and type C present more frequently with severe AR (30,33,34). For the sonographer, the main challenge is to find the “precise center” of the valve, which can sometimes differ from the geometrical center of the root. Multiplanar reconstruction helps to localize the coaptation center and to analyze the coaptation line up to the commissural edge by moving the short-axis plane (on the “en face view” plane) from the coaptation line to the end of the commissures. The starting point to measure the angle is the crossing point of the two other long-axis planes in the orthogonal plane (Figure 8A). Commissural orientation measurements can be seen as a “dynamic” MPR measurement. In addition, the more symmetric the BAV (type A), the more circular the annulus, while the more asymmetric the BAV (type B and type C), the more elliptic the annulus (17). The cH (mean: 4–5 mm) is the length of apposition of the cusp in diastole. The eH (mean: 8–10 mm) is the orthogonal distance from the annulus to the middle of the free margin of the cusp (Figure 8B). Systematic eH measurement allows the sonographer to detect a cusp prolapse. Due to the coaptation surface shape and the curvilinear aspect of the free margin, the cH and eH should not be measured on a single long-axis view of the AV. It should be noted that the maximal coaptation does not happen at the middle of the cusp (at the level of the Arantius nodule), but more laterally on the coaptation line. Conversely, the highest level of the cusp in diastole is on its middle, not at the level of the maximal coaptation. To calculate the true cH, the MPR plane must be perpendicular to the coaptation line on a short-axis view. Then, the cH is measured on the corresponding orthogonal view. To calculate the eH, the plane must be perpendicular to the cusp, from the bottom of the Valsalva to the opposite commissure (functional or not), or the opposite coaptation line. The FML is the free edge of the cusp between two commissures. It is measured on a short-axis view in diastole with MPR and a curvilinear tool (Figure 8C). The measured FML is a few millimeters shorter than the real structure, because it is a projection of the coaptation of a “semi-lunar” shape. An elongation of the FML can cause a cusp prolapse, and the FML is frequently elongated when the aortic root is dilated. The gH, also called the cusp height, is defined as the distance between the cusp nadir and the middle of the free margin, and it is measured using MPR in systole by crossing the free margin perpendicular to the middle of each cusp on the corresponding orthogonal plane (Figure 8D). The cusp is considered retracted when the gH is 16 mm or less in the TAV and 19 mm or less in the BAV non-fused cusp (8). The gH of the fused cusp and of the non-fused cusp tends to decrease from type A to type C.
Looking beyond the valve
The location of the coronary arteries must be identified by preoperative echocardiography in order to avoid a coronary injury during root dissection. For example, the right ostia can be very high, near the STJ. Two separate ostia emerging from the left sinus can also be observed (Figure 9) and may have implications on the surgical management. It is also advised to assess the pulmonary valve because in case of AVP failure, the surgeon may decide to convert to a Ross procedure. The pulmonary valve must have three semi-lunar cusps without significant regurgitation or stenosis. As for the AV, MPR helps to analyze the pulmonary valve. An asymmetric pulmonary root must be identified preoperatively. The transpulmonary gradient and a possible significant regurgitation are assessed with a mid-esophageal TEE view, usually between 60° and 90°.
Postoperative assessment of AVP—what is acceptable?
The main goal of post-AVP echocardiography, which must be performed in the operating room, is to identify factors associated with recurrent AR. Post-AVP assessment is challenging for the sonographer, because of the poor image quality due to air bubbles, acoustic shadowing coming from the aortic graft or from a retro-aortic hematoma, among others. The unstable hemodynamic state must also be taken into account when interpreting the transvalvular gradient and assessing a residual AR. The postoperative evaluation consists of four systematic steps (Table 4). The echocardiographic criteria associated with successful AVP and the criteria that are deemed unacceptable and require a second cardiopulmonary bypass run are presented in Table 5.
Table 4
| Steps | Observation(s) |
|---|---|
| Ventricular function and hemodynamics | If impaired, caution when interpreting transvalvular gradient and residual AR |
| Valve opening | Assess the presence of leaflet trill |
| Assess transvalvular gradient | |
| Assess color flow: laminar? | |
| Valve closure | Assess the level of cusp coaptation: should be above the aortic annulus |
| Exclude billowing or residual cusp prolapse | |
| Assess cH | |
| Assess eH | |
| Residual AR | Check eccentricity |
| Quantify |
AR, aortic regurgitation; cH, coaptation height; eH, effective height.
Table 5
| Accept | Questionable | Back on pump | |
|---|---|---|---|
| AR grade | Mild | > Mild | |
| AR jet | Central jet | Central jet due to overcorrection | Eccentric jet > trivial |
| Eccentric trivial jet | |||
| cH | ≥4 mm | <4 mm | |
| eH | ≥8 mm | <8 mm | |
| Transvalvular flow | Mean ≤15 mmHg | Infra- or supra-valvular | Mean >15 mmHg |
| Peak ≤30 mmHg | Peak >30 mmHg | ||
| Morphological aspects | Flexible cusp, end-systolic thrill | SRCM without gradient | Significant SRCM |
| TAV: no asymmetric commissural reimplantation | TAV: asymmetric commissural reimplantation without cusp prolapse | TAV: asymmetric commissural reimplantation with cusp prolapse | |
| Coaptation at the mid-level of the Valsalva sinuses | Billowing aspect | Residual prolapse |
AR, aortic regurgitation; cH, coaptation height; eH, effective height; SRCM, systolic restrictive cusp motion; TAV, tricuspid aortic valve.
Ventricular function and hemodynamics
In case of ventricular function impairment, the hemodynamics are compromised, leading to an underestimation of the transvalvular gradients and residual AR. Multiple transesophageal views centered on the left ventricle (at 0°, 60°, 90°, and 120°) and on the right ventricle (at 0° and 45°) are recommended. Similarly, a transgastric view at 0° is easy to obtain and provides a good evaluation of the global biventricular function in short axis. The postoperative ventricular function can be impaired secondary to ischemia (as a result of either pre-existing atherosclerosis, anatomical abnormalities, or trapped bubbles), severe left ventricular hypertrophy, or ineffective cardioplegia. Whether reimplanted or not, the coronary arteries can be damaged from surgically placed sutures within the aortic root. The echocardiographer should be aware of such possible injury and look for regional wall motion abnormalities and, if possible, visualize the blood flow in the proximal segments of the coronary arteries (21).
Valve opening
In case of compromised hemodynamics, the systolic leaflet motion and transvalvular flow cannot be adequately evaluated. Incomplete opening and early closure of the valve are indicative of low cardiac output and not necessarily of leaflet restriction. A little trill of the leaflet in end-systole appears as a good criterion for leaflet flexibility. On the short-axis view, the top of each cusp passing behind the virtual line between two commissures, near the bottom of the Valsalva, indicates that there is no SRCM. The transvalvular gradient and color flow through the LVOT are assessed by using a deep transgastric view. A postoperative mean gradient across the valve of 15 mmHg and a peak transvalvular gradient of 30 mmHg are deemed acceptable. Indeed, higher gradients are associated with an increased risk of developing severe aortic stenosis and redo operation. Postoperative correction of BAV should have a CO at 180° to decrease the postoperative systolic gradient (21,33).
Valve closure
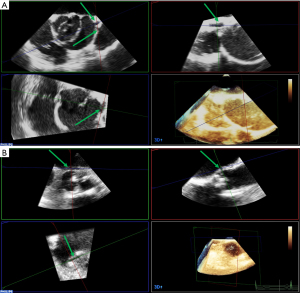
AVP surgery aims to obtain a functional coaptation by fully restoring the integrity of the entire FAA. Thus, the different components of the FAA must be assessed using postoperative echocardiography. The standard measurement of the repaired FAA can be performed on a simple 2D long-axis view and on MPR, as described above. Before aortic cross-clamp release, the effectiveness of the repair can be estimated by administering a terminal dose of blood cardioplegia into either the aortic root or tube graft. The root pressurization will oppose the cusps against each other (38). The non-pulsatile flow keeps the valve closed and helps to localize the origin and orientation of a residual jet (Figure 10A). It is an elegant technique, yet sometimes difficult to use. The commissures must be perfectly affixed against the aortic graft. On the short-axis view, a gap (frequently induced by a small hematoma) can be present between the two structures and can induce a commissural asymmetry, which can lead to a residual cusp prolapse. Another possible cause of cusp prolapse is the STJ over-reduction by the graft, thereby reducing the intercommissural distance. In any case, on the long-axis view, the level of cusp coaptation should be above the aortic annulus. This means that the lower level of the coaptation should be higher than the VAJ and its highest level should approach the mid-height of the sinuses of Valsalva (Figure 10B). If cusp coaptation occurs below the aortic annulus, the risk of recurrent AR is >70% (15,23). A billowing or a residual cusp prolapse must be carefully excluded. Geometric measurements are important to assess the quality of the AVP as well. The cH and eH are the most representative and should be compared to the pre-repair measurements. A cH less than 4 to 5 mm is associated with an increased risk of recurrent AR (>30–40%). The targeted eH must be 8 to 10 mm (15,39).
Quantification of a residual regurgitation
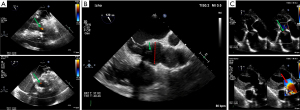
Evaluating the presence and direction of any residual AR is challenging in the immediate postoperative period but must be carried out by the imaging specialist because of its prognostic implications. The post-repair assessment uses the same criteria as the pre-repair assessment. If there is no residual AR and if coaptation is above the aortic annulus, recurrent AR is very unlikely. In any case, one should not accept more than a mild post-repair AR, especially if the AR is eccentric. Again, the hemodynamic state must be taken into account for the interpretation of residual AR severity. The deep transgastric view is the most appropriate to assess residual AR because it avoids acoustic shadowing (Figure 10C).
Conclusion and future perspectives
The AV and the aortic root are very complex structures. However, many regurgitant AVs can be repaired using current surgical techniques. Repair is possible for all AV morphological variants, including TAV, BAV, and, in some selected cases, UAV and QAV associated with AR. The AV can be preserved and repaired in most cases of isolated root aneurysm. This modern surgical management is still in its infancy and has a bright future, since it is associated with better long-term results than valve replacements, whether biological or mechanical. Of course, the implementation of this treatment is reserved to centers of expertise. The success of AVP is highly dependent on the discussion between the surgeon and the imaging specialist, who must share a common language. In this context, this review proposes a pre- and post-operative evaluation based on the systematic and detailed analysis of the aortic root and valve apparatus by TEE, and in particular by 3D volume-based analysis. An accurate and systematic analysis facilitates surgical planning. Currently, detailed exploration by 3D TEE with MPR remains the best way to evaluate the aortic root and regurgitant AV. However, sufficient training and expertise are mandatory to gain accurate and helpful clinical data. This review has an educational vocation and should contribute to disseminating the echocardiographic management of aortic root and valvular pathologies in order to achieve successful AVP. Given the sometimes suboptimal image quality and spatial resolution of echocardiography, other techniques such as CT-scan could also provide useful morphological information for planning complex AVPs in the future. This chapter has yet to be written.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35-71. Erratum in: Circulation 2021;143:e228 Erratum in: Circulation 2021;143:e784. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- David TE, David CM, Feindel CM, et al. Reimplantation of the aortic valve at 20 years. J Thorac Cardiovasc Surg 2017;153:232-8. [Crossref] [PubMed]
- Schäfers HJ, Raddatz A, Schmied W, et al. Reexamining remodeling. J Thorac Cardiovasc Surg 2015;149:S30-6. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, Vancraeynest D, et al. Late results of aortic valve repair for isolated severe aortic regurgitation. J Thorac Cardiovasc Surg 2021;S0022-5223(21)00612-7.
- Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring: from physiologic reconstruction of the root to a standardized approach for aortic valve repair. J Thorac Cardiovasc Surg 2010;140:S28-35; discussion S45-51. [Crossref] [PubMed]
- Lansac E, de Kerchove L. Aortic valve repair techniques: state of the art. Eur J Cardiothorac Surg 2018;53:1101-7. [Crossref] [PubMed]
- Bouhout I, Kalfa D, Shah A, et al. Surgical Management of Complex Aortic Valve Disease in Young Adults: Repair, Replacement, and Future Alternatives. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2022;25:28-37. [Crossref] [PubMed]
- Vanoverschelde JL, van Dyck M, Gerber B, et al. The role of echocardiography in aortic valve repair. Ann Cardiothorac Surg 2013;2:65-72. [PubMed]
- Abeln KB, Giebels C, Ehrlich T, et al. Which Aortic Valve Can Be Surgically Reconstructed? Curr Cardiol Rep 2021;23:108. [Crossref] [PubMed]
- Hagendorff A, Evangelista A, Fehske W, et al. Improvement in the Assessment of Aortic Valve and Aortic Aneurysm Repair by 3-Dimensional Echocardiography. JACC Cardiovasc Imaging 2019;12:2225-44. [Crossref] [PubMed]
- Tretter JT, Izawa Y, Spicer DE, et al. Understanding the Aortic Root Using Computed Tomographic Assessment: A Potential Pathway to Improved Customized Surgical Repair. Circ Cardiovasc Imaging 2021;14:e013134. [Crossref] [PubMed]
- Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010;11:645-58. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009;2:931-9. [Crossref] [PubMed]
- Regeer MV, Kamperidis V, Versteegh MIM, et al. Three-dimensional transoesophageal echocardiography of the aortic valve and root: changes in aortic root dilation and aortic regurgitation. Eur Heart J Cardiovasc Imaging 2017;18:1041-8. [Crossref] [PubMed]
- Hagendorff A, Stoebe S, Tayal B. A systematic approach to 3D echocardiographic assessment of the aortic root. Glob Cardiol Sci Pract 2018;2018:12. [Crossref] [PubMed]
- El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21. [Crossref] [PubMed]
- Mori S, Izawa Y, Shimoyama S, et al. Three-Dimensional Understanding of Complexity of the Aortic Root Anatomy as the Basis of Routine Two-Dimensional Echocardiographic Measurements. Circ J 2019;83:2320-3. [Crossref] [PubMed]
- Underwood MJ, El Khoury G, Deronck D, et al. The aortic root: structure, function, and surgical reconstruction. Heart 2000;83:376-80. [Crossref] [PubMed]
- Van Dyck MJ, Watremez C, Boodhwani M, et al. Transesophageal echocardiographic evaluation during aortic valve repair surgery. Anesth Analg 2010;111:59-70. [Crossref] [PubMed]
- El Khoury G, Vanoverschelde JL, Glineur D, et al. Repair of bicuspid aortic valves in patients with aortic regurgitation. Circulation 2006;114:I610-6. [Crossref] [PubMed]
- Jeanmart H, de Kerchove L, Glineur D, et al. Aortic valve repair: the functional approach to leaflet prolapse and valve-sparing surgery. Ann Thorac Surg 2007;83:S746-51; discussion S785-90. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007;116:I264-I269. [Crossref] [PubMed]
- Schneider U, Hofmann C, Schöpe J, et al. Long-term Results of Differentiated Anatomic Reconstruction of Bicuspid Aortic Valves. JAMA Cardiol 2020;5:1366-73. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Sillesen AS, Vøgg O, Pihl C, et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021;325:561-7. [Crossref] [PubMed]
- Michelena HI. Speaking a common language: the international consensus on bicuspid aortic valve nomenclature and classification. Ann Cardiothorac Surg 2022;11:402-17. [Crossref] [PubMed]
- Hurwitz LE, Roberts WC. Quadricuspid semilunar valve. Am J Cardiol 1973;31:623-6. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg 2021;60:448-76. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Chirichilli I, Irace FG, Weltert LP, et al. A direct correlation between commissural orientation and annular shape in bicuspid aortic valves: a new anatomical and computed tomography classification. Interact Cardiovasc Thorac Surg 2020;30:666-70. [Crossref] [PubMed]
- Ehrlich T, de Kerchove L, Vojacek J, et al. State-of-the art bicuspid aortic valve repair in 2020. Prog Cardiovasc Dis 2020;63:457-64. [Crossref] [PubMed]
- de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019;ezz033. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- Detaint D, Messika-Zeitoun D, Maalouf J, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging 2008;1:1-11. [Crossref] [PubMed]
- Boodhwani M, de Kerchove L, Glineur D, et al. A simple method for the quantification and correction of aortic cusp prolapse by means of free margin plication. J Thorac Cardiovasc Surg 2010;139:1075-7. [Crossref] [PubMed]
- Koshy T, Misra S, Sinha PK, et al. A novel technique to assess aortic valve repair before releasing the aortic cross-clamp. J Cardiothorac Vasc Anesth 2009;23:79-81. [Crossref] [PubMed]
- Pethig K, Milz A, Hagl C, et al. Aortic valve reimplantation in ascending aortic aneurysm: risk factors for early valve failure. Ann Thorac Surg 2002;73:29-33. [Crossref] [PubMed]






