Reimplantation of the aortic valve in patients with tricuspid aortic valve: the Toronto General Hospital experience
Introduction
Over 30 years ago we published a series of ten patients with aortic root aneurysms treated with a new procedure developed to preserve the native aortic valve which was named aortic valve sparing operation (1). Three years later we classified aortic valve sparing operations in two basic types: reimplantation of the aortic valve (RAV) and remodeling of the aortic root (2). Prior to those reports, the surgical treatment of aortic root aneurysms whether it was associated with normal or abnormal aortic valve, was to replace the aortic root with a valved-conduit using either a mechanical or a tissue valve, along with reimplantation of the coronary arteries. While this operation can be applied to all patients, in the case of aneurysm with normal or incompetent aortic valve [aortic insufficiency (AI)], an aortic valve sparing operation may be preferable (3). We have been following all patients who have had aortic valve sparing operations at our hospital, and herewith report the late clinical outcomes of patients with aortic root aneurysms and a tricuspid aortic valve who were treated with RAV.
Methods
Four hundred and four consecutive patients had RAV from 1989 through 2019 at Toronto General Hospital. The operative technique has been described in previous publications (1,2,4). The size of the graft used for RAV was determined based on the average height of the cusps as originally described (1). Approximately two-thirds of patients had the valve implanted into a straight tubular Dacron. The remaining one-third had the valve implanted in a graft 2 to 3 mm larger than estimated and plicated at the sub-annular level to reduce its diameter to the estimated size and after reimplanting the valve, the intercommissural distance was reduced by plication of the graft at the level of the new sinotubular junction. No patient had commercially available Valsalva grafts. Cusps with large fenestrations had the free margins reinforced with a double layer of a fine expanded polytetrafluoroethylene suture (5). Prolapsing cusps were shortened by plication of the free margins along the nodule of Arantius. Pericardial patches were not used to augment or repair defective cusps.
Patients were followed prospectively with periodic assessment of aortic valve function and imaging of the thoracic and abdominal aorta. Echocardiograms were obtained in the operating room, before discharge from the hospital and every two to five years, depending on where the patient lived. This study was approved by the Review Ethics Board of University Health Network, and written consent was required from all patients.
Statistical analysis
Clinical characteristics were summarized using median [interquartile range (IQR)] for continuous variables and frequencies for dichotomous and polytomous variables. Post-operative mortality was estimated using the Kaplan-Meier survival method. Valve-related reoperations in the aortic valve, development of post-operative moderate and severe AI and new aortic dissections were characterized using competing risk models in terms of cumulative incidence function. Administrative censoring at 20 years was applied to all time-to-event analyses. Cox proportional hazard regression was applied to assess and quantify the association of risk factors with mortality in terms of hazard ratios (HRs). The corresponding 95% confidence intervals (CIs) and P values were evaluated using Wald’s statistics. The following variables were entered into the Cox regression: patient’s age at the time of surgery, acute or chronic dissections, moderate and severe preoperative AI, cusp repair with expanded polytetrafluoroethylene suture, cusp plication and creation of neo-aortic sinuses. Fine-Gray competing risk models were separately applied to assess and quantify the association of risk factors with valve-related reoperation and the development of moderate or severe AI. The corresponding 95% CIs and P values were evaluated using Wald’s statistics. Multiple imputation using chained equation (MICE) was employed for missing values. The aforementioned regression analyses were separately conducted on each imputed data, and the results were then combined using Rubin’s rule. Analyses were performed using R [Core Team (2020): A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria].
Results
Table 1 summarizes the clinical profile of the patients. The median age was 48.0 (IQR, 35.0–59.0) years, and 310 (76.7%) were men. There were 150 patients with Marfan syndrome and 20 with Loeys-Dietz syndrome. Table 2 shows information on the operative procedures performed. Eleven patients were lost to follow-up from one to 12 years after surgery. There were 55 patients alive and free from reoperation at 20 years. The median follow-up was 11.7 (IQR, 6.8–17.1) years.
Table 1
| Variables | Values (n=404) |
|---|---|
| Age, median [IQR] (years) | 48.0 [35.0–59.0] |
| Sex: men | 310 (76.7) |
| Electrocardiogram | |
| Sinus rhythm | 395 (97.7) |
| Atrial fibrillation | 8 (2) |
| Heart block/pacemaker | 1 (0.2) |
| Previous cardiac surgery | |
| Ross procedure | 2 (0.5) |
| Mitral valve repair | 4 (1.0) |
| Heart transplant | 1 (0.2) |
| Other | 10 (2.5) |
| Marfan syndrome | 150 (37.1) |
| Loeys-Dietz syndrome | 20 (5.0) |
| Preoperative aortic dissections | 33 (8.2) |
| Associated disorders | |
| Diabetes mellitus | 17 (4.2) |
| Hypertension | 155 (38.4) |
| Hyperlipidemia | 90 (22.3) |
| COPD (FEV1 <1) | 7 (1.7) |
| Previous stroke | 8 (2) |
| Peripheral vascular disease | 6 (1.5) |
| Renal failure (dialysis) | 5 (1.2) |
| Remote infective endocarditis | 5 (1.2) |
| Coronary artery disease (n=355) | 41 (11.5) |
| New York Heart Association functional class (n=393) | |
| Class I | 261 (66.4) |
| Class II | 83 (21.1) |
| Class III | 24 (6.1) |
| Class IV | 25 (6.4) |
| Left ventricular ejection fraction (n=393) | |
| ≥60% | 281 (71.5) |
| 40–59% | 94 (23.9) |
| 20–39% | 18 (4.6) |
| Preoperative echocardiography (n=385) | |
| Aortic insufficiency | |
| None, trivial and mild | 222 (57.7) |
| Moderate | 94 (24.4) |
| Severe | 69 (17.9) |
| Mitral regurgitation (moderate/severe) | 29 (7.5) |
| Tricuspid regurgitation (moderate/severe) | 2 (0.5) |
| Atrial septal defect | 25 (6.5) |
| Ventricular septal defect | 3 (0.8) |
| Aortic root diameter, median [IQR] (mm) | 53 [50–55] |
| Aortic annulus diameter, median [IQR] (mm) | 29 [27–30] |
Percentages are shown in parentheses unless indicated as IQR. IQR, interquartile range; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second.
Table 2
| Variables | Values |
|---|---|
| Size of graft, median [IQR] (mm) | 30 [28–32] |
| Aortic cusp plication | 131 (32.4) |
| Free margin reinforcement with Gore-Tex | 99 (24.5) |
| Creation of neo-aortic sinuses | 134 (33.1) |
| Mitral valve repair | 38 (8.1) |
| Mitral valve replacement | 1 (0.2) |
| Tricuspid annuloplasty | 2 (0.4) |
| Coronary artery bypass | 40 (9.9) |
| Replacement of aortic arch/hemiarch | 46 (11.3) |
| Closure of atrial septal defect | 25 (6.1) |
| Closure of ventricular septal defect | 3 (0.7) |
| Maze procedure | 6 (1.5) |
| Repair of abdominal aortic aneurysm | 1 (0.2) |
| Cardiopulmonary bypass time, median [IQR] (min) | 138 [117–164] |
| Aortic clamping time, median [IQR] (min) | 114 [98–136] |
Percentages are shown in parentheses unless indicated as IQR. IQR, interquartile range.
Perioperative complications
There were five early deaths (either in hospital or within 30 days) and the following non-fatal complications: re-exploration of the chest for bleeding/tamponade/cardiac arrest in 29 (7.2%) patients, aortic root replacement with a mechanical valve on the second postoperative day for persistent AI in one patient, mitral valve repair for perforation of the anterior leaflet in one patient and for systolic anterior motion of the anterior leaflet of the mitral valve in one patient, repair of liver rupture caused by resuscitation for cardiac arrest in one patient, permanent transvenous pacemaker in eight (2%) patients, myocardial infarction in five patients, postoperative new atrial fibrillation in 92 (23%) patients, transient ischemic attack in two patients, stroke in three patients, sepsis with positive blood culture in eight (2%) patients, and transfusion of blood products in 217 (54%) patients. The median hospital stay was six (IQR, 5–8) days.
Late mortality
There were 56 late deaths: 14 cardiovascular and 42 non-cardiovascular-related. Table 3 shows the cumulative incidence of mortality at various time intervals. Multivariable analysis identified the following variables associated with overall mortality: age [HR =1.05 (95% CI: 1.03, 1.08), P<0.001], aortic dissection [HR =3.51 (95% CI: 1.31, 9.48), P=0.01] and left ventricular ejection fraction <40% [HR =2.39 (95% CI: 1.05, 5.45), P=0.03].
Table 3
| Variables | Time | ||
|---|---|---|---|
| 1 year | 10 years | 20 years | |
| Death from any cause | 2.5 [1.3, 4.6] | 9.6 [6.9, 13.3] | 26.7 [20.6, 34.2] |
| Aortic valve reoperation | 0.7 [0.2, 2.3] | 1.9 [0.9, 4.1] | 7.0 [4.0, 12.2] |
| Moderate/severe AI | 1.2 [0.5, 3.0] | 8.7 [6.2, 12.3] | 11.8 [8.5, 16.5] |
| Thromboembolism | 1.5 [0.7, 3.3] | 6.3 [4.2, 9.4] | 9.5 [6.4, 14.2] |
| Endocarditis | 0.0 | 0.5 [0.1, 2.2] | 1.6 [0.4, 6.0] |
| Pacemaker implantation | 2.2 [1.2, 4.3] | 5.4 [3.5, 8.3] | 7.2 [4.6, 11.3] |
| New distal aortic dissections | 1.0 [0.4, 2.6] | 3.4 [1.9, 5.9] | 13.2 [8.9, 19.6] |
All values are given in percentages; values in brackets are the 95% CI. AI, aortic insufficiency; CI, confidence interval.
Cardiovascular reoperations
Aortic valve reoperation was performed in 19 (4.7%) patients from two to 27 years after surgery. One patient had aortic cusp repair and 18 had aortic valve replacement. One patient died at reoperation. The indication for reoperation in the aortic valve was moderate or severe AI in 16 (84%) patients and infective endocarditis in three (16%) patients. Table 3 show the cumulative incidence of reoperations on the aortic valve over time. Figure 1 shows survival free from reoperation and the cumulative incidence of reoperation over time. We did not identify any variable associated with reoperation on the aortic valve by multivariable analysis.
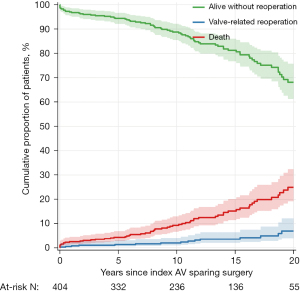
In addition to reoperations on the aortic valve, seven patients required mitral valve repair, five patients had total arch replacement with elephant trunk, seven patients had replacement of entire thoracic and abdominal aorta, three patients had replacement of the descending thoracic aorta, four patients had replacement of the abdominal aorta, six patients had endovascular stenting of the thoracic (five patients) or abdominal aorta (one patient), and three patients had iliac arteries aneurysm repaired with open surgery. All but three patients who had arch or distal aortic surgery had had aortic dissection either before or after the index operation.
Aortic valve dysfunction
Forty patients developed moderate or severe AI during follow-up. Table 3 show the cumulative incidences of AI at various times intervals and Figure 2 shows it over time as well as survival free from AI. One patient developed calcific aortic stenosis 24 years after surgery with a mean systolic gradient of 34 mmHg at the most recent echocardiogram. We could not identify any variable associated with the development of AI by multivariable analysis.
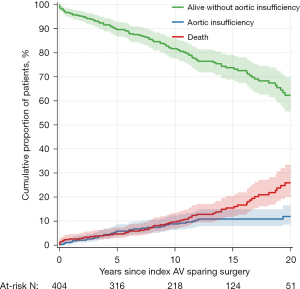
Other valve-related events
Thromboembolism was documented in 30 patients: transient ischemic attack in 20 and stroke in ten. Two patients died after suffering a stroke. Table 3 shows the cumulative incidence of thromboembolism at various times intervals. Three patients developed infective endocarditis; all three required aortic root replacement with aortic homograft and survived. Table 3 shows the incidence of infective endocarditis at various times intervals. Eight patients required a permanent transvenous pacemaker in hospital after surgery and 17 patients required a permanent transvenous pacemaker during the follow-up. Table 3 shows the incidence of implanted pacemaker at various times intervals. New distal aortic dissections (arch and descending thoracic aorta) occurred in 31 patients during follow up, 27 of these patients had known genetic syndromes. Table 3 shows the cumulative incidence of new aortic dissections at various times intervals. Figure 3 shows the proportion of patients free from dissection and the cumulative incidence of new dissections over time.
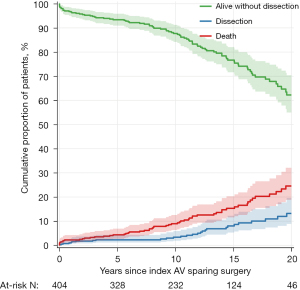
Discussion
The usefulness of RAV to treat patients with aortic root aneurysms with a tricuspid aortic valve has been demonstrated in this series of patients as well as in previous reports from Toronto General Hospital (5,6). A cumulative mortality of 27% at 20 years in our patients may appear higher than expected because their median age was only 48 years at the time of surgery. One would expect a higher survival rate but a large proportion of our patients had aortic root aneurysms associated with genetic syndromes and suffered pre- as well as postoperative aortic dissections and other associated disorders such as pulmonary complications, affecting late survival. Advancing age, preoperative aortic dissection and impaired left ventricular function were associated with late mortality as indicated by multivariable regression analysis in this report.
Only 7% of the patients required reoperation on the aortic valve after 20 years of follow-up and we had 55 patients at risk at that time interval. This reoperation rate is slightly lower than that reported by others (5-10). This may be due to patient selection and the fact that most operations in this series were performed by two experienced surgeons in heart valve reconstruction. Most reports indicate an incidence of 10% to 15% at 10 to 15 years (7-10). In addition to reoperations on the aortic valve, these patients frequently require distal aortic operations, particularly if they had aortic dissections preoperatively or developed aortic dissections postoperatively. Those with genetic syndromes are more likely to experience aortic dissections during the follow-up. Further aortic operations in these patients are common and even more common than reoperation on the aortic valve. The development of mitral regurgitation was another cause of reoperation in our series but again, more common in patients with associated genetic syndromes.
The Achilles’ heel of aortic valve sparing operations in patients with tricuspid aortic valves is the development of AI. This lesion is often well tolerated and may be underdiagnosed unless patients are monitored with echocardiography. Therefore, as with other types of heart valve repairs, freedom from reoperation is not the same as freedom from valve failure. In this series of RAV, the incidence of moderate and severe AI was almost twice as high as the incidence of reoperations on the aortic valve. The longer one follows these patients the more aortic valve dysfunction will be detected (6). In addition to time since surgery, other variables are likely associated with the development of moderate or severe AI over time. We examined multiple variables but we could not identify any association by multivariable analysis, likely due to incomplete data on the morphology of the reconstructed aortic root such as diameters of the aortic annulus, cusps coaptation height and coaptation length. Preoperative AI of moderate or severe degree was not associated with the development of postoperative AI by multivariable analysis but it was by univariable analysis. With more patients at risk and with longer follow-up, we may detect more variables associated with valve failure. We also carefully examined the role of the creation of neo-aortic sinuses and its effect on the development of AI, which showed benefit in some analyses, but could not confirm that it played a role in the development of AI due to missing data and probable inappropriate statistical modeling. One-third of our patients had neo-aortic sinuses created using a graft one size larger than needed and plicating the graft beneath the nadir of the scalloped aortic annulus and, after the valve was reimplanted, plicating the area in between two commissures at the level of new sinotubular junction as described in previous publications (11). This variable was examined but due to incomplete data we could not confirm if this technique is beneficial or not. We plan to review all intraoperative echocardiograms and to obtain accurate information regarding geometry of the reconstructed root (a difficult task because the experience spans over three decades) and then re-analyze the data with all variables known to affect the development of postoperative AI, including surgeons’ experience.
As it happens in every area of medicine, the longer we follow patients who receive treatment for a given disorder, the more we learn about it. This present study underscores the need of lifelong surveillance of patients with aortic root aneurysms, particularly if they are associated with known genetic syndromes because the risk of new aortic dissection and its complications is relatively high. In addition, these patients should be cared for in centers of excellence with the capability of caring for all their needs, not only cardiovascular.
In conclusion, RAV continues to provide very good clinical outcomes and aortic valve function remains stable in most patients during the first two decades of follow-up when correctly performed.
Acknowledgments
Funding: This project was sponsored by a generous donation the Division of Cardiovascular Surgery Research Fund by Mr. Todd Halpern.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [Crossref] [PubMed]
- David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg 1995;109:345-51; discussion 351-2. [Crossref] [PubMed]
- Ouzounian M, Rao V, Manlhiot C, et al. Valve-Sparing Root Replacement Compared With Composite Valve Graft Procedures in Patients With Aortic Root Dilation. J Am Coll Cardiol 2016;68:1838-47. [Crossref] [PubMed]
- David TE. Remodeling of the aortic root and preservation of the native aortic valve. Operative Techniques in Cardiac & Thoracic Surgery 1996;1:44-56. [Crossref]
- David TE, Seidman MA, David CM, et al. Outcomes of Reimplantation of the Aortic Valve in Patients With Aortic Cusp Fenestration. Ann Thorac Surg 2023;115:106-11. [Crossref] [PubMed]
- David TE, David CM, Ouzounian M, et al. A progress report on reimplantation of the aortic valve. J Thorac Cardiovasc Surg 2021;161:890-899.e1. [Crossref] [PubMed]
- Beckmann E, Martens A, Krüger H, et al. Aortic valve-sparing root replacement with Tirone E. David's reimplantation technique: single-centre 25-year experience. Eur J Cardiothorac Surg 2021;60:642-8. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, Lemaire G, et al. Two decades of valve-sparing root reimplantation in tricuspid aortic valve: impact of aortic regurgitation and cusp repair. Eur J Cardiothorac Surg 2021;59:1069-76. [Crossref] [PubMed]
- Klotz S, Stock S, Sievers HH, et al. Survival and reoperation pattern after 20 years of experience with aortic valve-sparing root replacement in patients with tricuspid and bicuspid valves. J Thorac Cardiovasc Surg 2018;155:1403-1411.e1. [Crossref] [PubMed]
- Fraser CD 3rd, Liu RH, Zhou X, et al. Valve-sparing aortic root replacement in children: Outcomes from 100 consecutive cases. J Thorac Cardiovasc Surg 2019;157:1100-9. [Crossref] [PubMed]
- David T. Reimplantation valve-sparing aortic root replacement is the most durable approach to facilitate aortic valve repair. JTCVS Tech 2021;7:72-8. [Crossref] [PubMed]






