The perioperative outcomes of uniportal robotic-assisted thoracic surgeries—a systematic review and meta-analysis of surgical cohort studies and case reports
Introduction
Following the mainstream adoption of robotic-assisted surgical approaches in most surgical subspecialties, cardiothoracic surgery has too seen the integration and recognition of robotic-assisted thoracoscopic surgery (RATS) as the ‘cutting edge’ of the field. With excellent rates of technical success and oncologic efficacy of uniportal video-assisted thoracoscopic surgery (u-VATS) established, uniportal robotic-assisted thoracic surgery (u-RATS) is now also being developed for appropriate patients and pathologies, all in keeping with the philosophy of offering patients maximal efficacy treatments with minimal invasiveness and trauma (1,2). At the time of writing, however, there is no current, coherent body of evidence exploring the outcomes of these patients in the early phase of adoption of u-RATS, given the first cases undergoing this procedure were only completed in late 2021. This systematic review and meta-analysis hence sought to aggregate the current experimental evidence and illustrate the outcomes for patients undergoing u-RATS approaches in cohort studies and case reports.
Methods
Literature search strategy
The methods for this systematic review adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) updated statement (3). Four electronic databases were used to perform the literature searches, encompassing EMBASE, Ovid MEDLINE, PubMed, and SCOPUS. These databases were searched from the date of database inception through to February 2023. For the examination of the outcomes of the u-RATS approach, a search strategy using the combination of keywords and Medical Subject Headings (MeSH) including (uniportal AND robotic AND lobectomy) OR (thoracic surgery) was utilized and is visually presented by the PRISMA flow diagram (see Figure S1). Predefined selection criteria were applied to assess for inclusion (see “Inclusion and exclusion criteria”). Each study was screened independently by two co-authors, with any conflicts resolved prior to progression through mutual agreement. Where the title and/or abstract provided insufficient detail in the determination of relevance for additional screening, a full-text review of the record was carried out in the first instance.
Inclusion and exclusion criteria
Studies were included in the review if they examined the perioperative and postoperative outcomes of interest in patients undergoing u-RATS procedures (see “Primary and secondary endpoints”). Series remained eligible for inclusion in the instance of ‘hybrid’ approaches where VATS instrumentation was used in the absence of RATS instrumentation due to logistical constraints/non-access. Studies were excluded for: (I) non-English reporting; (II) narrative reports (case reports of 1 were included given the paucity of literature and minimal patient volumes on this topic); (III) studies without clear recruiting details; (IV) no mention of perioperative and postoperative patient outcomes. Reference lists of the included studies were reviewed at completion of the database search to identify any extra, relevant studies not already included.
Primary and secondary endpoints
The primary endpoint of analysis was technical success as defined by surgical completion of the operation without conversion to u-VATS or open. The secondary endpoints of analysis included blood loss, chest tube duration, length of stay (LOS), lymph nodes retrieved (with station details if included), number of cases for learning curve (if reported), and other perioperative and postoperative details
Data extraction, critical appraisal, and quality assessment
Two independent reviewers extracted data directly from publication texts, tables, and figures. A third reviewer independently reviewed and confirmed all extracted data. Differing opinions between the two main reviewers were resolved through discussion led by the primary investigator. Attempts were made to clarify insufficient/indistinct data from authors of included studies, as required. Data was extracted in a way that each study was effectively treated as a case series, irrespective of underlying design. The Canadian Institute of Health Economics Quality Appraisal score was used as the quality assessment tool (4). Studies were defined as low quality with scores ≤10/19, moderate quality 11–15/19, and high quality >15/19.
Statistics
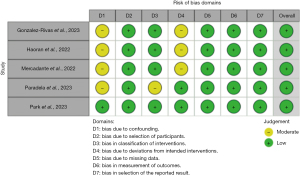
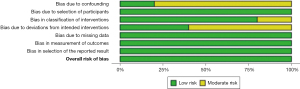
A meta-analysis of proportions or means were performed for categorical and continuous variables, as appropriate, by an independent reviewer. A random effects model was used to account for differing regions, surgeon experience, surgical technique and equipment, and management protocols across the included studies. Means and standard deviations were calculated from the median, where reported, using the methods described by Wan and colleagues (5). Pooled data and standard deviations (SD) are presented as N (%) ± SD with 95% confidence intervals (CI). For outcome data, heterogeneity amongst studies was assessed using the I2 statistic. Thresholds for these values were considered as low, moderate, and high heterogeneity as 0–49%, 50–75% and greater than or equal to 75%, respectively. Meta-analysis of proportions or means were performed using Stata (version 17.0, StataCorp, Texas, USA). Risk of bias was assessed using the “Risk of Bias in Non-randomized Studies - of Interventions” (ROBINS-I) tool and has been visually presented (see Figures 1,2) (6). Reporting of individual variables is also noted. Funnel plots were generated using R {R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Studio [RStudio Team (2020)]} in the R Studio environment (RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, USA), with Egger’s and Begg’s tests applied for assessment of small-study effects and publication bias.
Results
Study characteristics and baseline demographic data (seeTables 1,2)
Table 1
| Characteristics | Cohort studies (studies reported; total cases) | Value | 95% confidence interval |
|---|---|---|---|
| Cohort size (n; mean per study) | 5/5; 233/233 | 233 (62.6) | 58.4–66.9 |
| Males (n; mean) | 5/5; 126/126 | 126 (35.5) | 32.7–38.2 |
| Mean age of cohort (years ± SD) | 5/5; 233/233 | 59.7±3.0 | 59.3–60.1 |
| Mean FEV1 pre-op (L/s ± SD) | 2/5; 130/233 | 84.8±3.2 | 84.2–85.3 |
| Mean tumor size (cm2 ± SD) | 3/5; 93/233 | 3.2±0.8 | 3.1–4.0 |
| Mean operation time (min ± SD) | 5/5; 233/233 | 133.8±38.2 | 128.9–138.8 |
| Mean blood loss (mL ± SD) | 5/5; 233/233 | 80.0±25.1 | 50.1–100.3 |
| Mean chest tube time (days ± SD) | 4/5; 209/233 | 2.7±1.1 | 2.5–2.8 |
| Mean length of stay (days ± SD) | 5/5; 233/233 | 4.4±1.6 | 2.8–6.0 |
| Mean conversion to open procedure (%) | 5/5; 233/233 | 0.04 | NA |
n, number; SD, standard deviation; FEV1, forced expiratory volume 1 second; NA, not applicable.
Table 2
| Characteristics | Value | 95% confidence interval |
|---|---|---|
| Cohort size (n) | 7 | NA |
| Males (n) | 4 | NA |
| Mean age of cohort (years ± SD) | 58.1±6.8 | 41.5–74.8 |
| Mean FEV1 pre-op (L/s ± SD) | 90.0±27.6 | 46.0–133.6 |
| Mean tumor size (cm2 ± SD) | 2.8±0.9 | 1.7–4.0 |
| Mean operation time (min ± SD) | 150.0±52.2 | 20.3–279.7 |
| Mean chest tube time (days ± SD) | 3.7±0.6 | 2.2–5.1 |
| Mean length of stay (days ± SD) | 4.3±1.1 | 3.3–5.3 |
| Mean conversion to open procedure (n ± SD) | 0 | NA |
n, number; SD, standard deviation; FEV1, forced expiratory volume 1 second; NA, not applicable.
A total of 137 studies on the application of u-RATS were identified for inclusion, with 12 progressing to inclusion (5 cohort series, 7 case reports) (7-18). The cohort series are presented in Table 3. Two hundred and forty patients were collectively involved (233 from cohort studies, 7 from case reports). Fifty-two percent were male. Three of the cohort studies were drawn from Europe (Spain, Portugal, Italy) and two were drawn from Asia (China, Japan). Five case reports were drawn from Europe (Spain, Italy) and two were drawn from China. On quality assessment of the cohort group, one study was of high quality, three were of moderate quality, and one was of low quality. Technical success (as defined by the primary endpoint of interest) was 99.9%.
Table 3
| Principal author | Year [recruited] | Location | Cohort | Procedures |
|---|---|---|---|---|
| Haoran et al. | 2022 [2022] | China | 40 | u-RATS lobectomies |
| Gonzalez-Rivas et al. | 2023 [2021] | Spina | 30 | u-RATS sleeves, lobectomies |
| Paradela et al. | 2023 [2022] | Portugal | 100 | u-RATS anatomic segmentectomies, sleeves |
| Mercadante et al. | 2022 [2022] | Italy | 24 | u-RATS lobectomies |
| Park et al. | 2023 [2020] | Korea | 39 | u-RATS anterior mediastinal resections |
u-RATS, uniportal robotic-assisted thoracic surgery.
The mean age of patients in the cohort group was 59.7±3.0 years. The mean forced expiratory volume in one second (FEV1) pre-operatively was 84.8±3.2 L. The mean tumor size was 3.2±0.8 cm2. The mean operation time was 133.8±38.2 minutes. The mean blood loss was 80.0±25.1 mL. The mean chest tube duration was 2.7±1.1 days. The mean LOS was 4.4±1.6 days. Conversion from the u-RATS approach occurred in 1 patient. The mean age of patients in the case report group was 58.1±6.8 years. The mean FEV1 pre-operatively was 90.0±27.6. The mean tumor size was 2.8±0.9 cm2. The mean operative time was 150.0±52.2 minutes. The mean chest tube duration was 3.7±0.6 days. The mean LOS was 4.3±1.1. Only 1 patient required conversion, and it was to biportal RATS. Overwhelmingly, the procedures completed in both groups were lobectomies/anatomic resections, though mediastinal masses were also identified to be amenable to this approach. Outcomes of interest that were not reported (or were not reported sufficiently for meta-analysis) were namely: renal impairment, hypertension, diabetes, chronic obstructive pulmonary disease, atrial fibrillation, peripheral vascular disease, coronary artery disease, prior acute coronary syndrome or myocardial infarction, cerebrovascular accident or transient ischemic attack, reoperation and readmission (30-day).
Risk of bias assessment
All 5 included cohort studies (7,8,11,14,15) were deemed to be of low risk of bias, with the primary concern being the lack of control arms and potential for selection bias given strict selection criteria of the patients for the series.
Assessment of publication bias
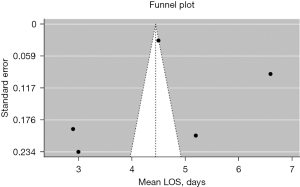
A review of the Prospective Register of Systematic Reviews (PROSPERO) demonstrated that no other existing systematic reviews have been registered on this topic as of March 2023. Funnel plot assessment of the mean operating time, blood loss, and LOS, along with Egger’s and Begg’s test of mean operating time and LOS, respectively, suggest no strong evidence of funnel plot asymmetry, publication bias, or small-study effects (see Figures 3-5). Given the small sample sizes, the range of datapoints for the funnel plots was significant, as can be appreciated (see Figures 3-5).

Discussion
Following the mainstream adoption of robotic thoracic surgery in international centers of excellence, leaders in the field have continued to develop increasingly less invasive approaches such as the biportal, and now the uniportal approach for amenable pathologies, with excellent early outcomes; however, no collated data on this topic currently exist given the early phase nature of this technique (19). Studies are currently underway directly comparing multiportal RATS to uniportal RATS, though these results are yet to be published. The aim of the present systematic review and meta-analysis was to therefore provide the most up-to-date, comprehensive assessment of the early literature on uniportal robotic thoracic surgeries, primarily to determine its feasibility (i.e., technical success and operation times) and core metrics of perioperative safety (i.e., blood loss, chest tube duration, and LOS).
Overwhelmingly, the included studies focused on the application of u-RATS for lobectomies, segmentectomies, and wedge resections, though some authors have also accomplished proficiency in the more complex sleeve resections (8,13), to good effect. Anterior mediastinal masses also appear readily amenable to this approach, with slight variations on the technique required to optimize robotic arm/camera positioning (15). A critical issue in establishing the utility and long-term viability of u-RATS is its oncologic efficacy—prior studies have highlighted that standard multiportal robotic surgical approaches have improved oncologic outcomes with respect to increased lymph node excision (i.e., number of total nodes and stations sampled), nodal upstaging, and reduced morbidity factors such as blood loss, with the findings of this review supporting the latter with a mean of 80±25.1 mL volume loss collectively in the cohort group. Unfortunately, no meta-analysis on lymph node removal was possible due to sparse reporting.
Another key point of criticism is whether the uniportal RATS approach, and even the multiportal RATS approach, can confer a significant benefit over that of the uniportal VATS approach when procedures are completed in expert hands. Comparative head-to-head studies examining multiportal RATS vs. u-RATS are soon to have their results published, which will assist with differentiating between these modalities and their outcomes (8,20,21). Whilst the studies included in this meta-analysis have variations of procedures—and pathology to a lesser extent—the overarching aim of the present paper was to illustrate that irrespective of the specific procedure being completed, operations can be conducted safely, quickly, and with very high rates of technical success.
Technical challenges, advantages, and lessons learned in transitioning to u-RATS
As the robotic platform was designed with a multiportal approach in mind, the recent development of single port specialized, multi-articular, flexible instrumentation has allowed for even more precise and meticulous dissection without compromising on degrees of freedom in the confined u-RATS space (22). These instruments remain limited and center-specific, with other centers having to use VATS, standard multiportal or jerry-rigged instrumentation (e.g., transoral trocars) as surrogates (7). The advent of the dedicated single port Da Vinci patient cart and trocars (Intuitive Surgical Inc., Sunnyvale, California, USA) will likely close this gap further, though cost remains a critical factor—instead of utilizing a modified multiportal system, an entire new system must be bought (23). Utilizing the standard system is much more cost-efficient in this respect, though the tradeoff is careful attention to arm positioning and transitioning at times to mitigate collision throughout different phases of each operation.
The three-dimensional view and tremor mitigation, intrinsic benefits of any robotic platform but particularly the standard multiportal Da Vinci system, further facilitates careful dissection in the u-RATS environment. Most centers specializing in u-RATS procedures have now also established efficient and standardized procedural steps to facilitate rapid arm docking, transition between instruments, and ultimately a faster dissection/resection time—though notably, an experienced surgical assistant is required as they will usually be responsible for approximately half of the operation (i.e., being by the bedside, docking/undocking, generating the utility incision, etc.) (21). A clear recommendation across the included studies is that the baseline level of operator experience has to therefore be high with uniportal VATS prior to making the transition across to biportal and uniportal RATS, as the learning curve is likely to be significantly steeper (24), and all parties must be ready in the instance of an emergency to convert.
Limitations
There are a number of limitations in the present systematic review that are critical to highlight. Reporting of key comorbidities, such as smoking status or past medical history, was universally poor across the included studies, with only one author partially detailing these datapoints (11). Surprisingly, only two of the included studies reported workable information on lymph node clearance, prohibiting meta-analysis, despite this being a fundamental datapoint in establishing the utility of this surgical approach (7,14). Margin clearance was notably also not reported. It must also be made clear that almost all patients in this series notably received operations from technical experts (defined as operators with an established history of multiportal, uniportal VATS and RATS and industry recognition of a high-degree of technical skill), with exceedingly low rates of conversion to either open or secondary RATS/VATS approaches (i.e., biportal/multiportal). These results are alongside fast operating times, minimal blood loss, and rapid discharge from hospital. As these patients were carefully enrolled for the series with respect to having amenable pathologies, the inherent risk of selection bias applies. The risk of bias assessment included in this study identified a low overall risk, however. Pathology/approach-specific procedure time was not able to be accounted for given their aggregation within each study, though the primary point of illustration in this review is to highlight that in expert hands, procedural times are well within acceptable margins. No strong evidence of publication bias was detected on Funnel plot assessment, Egger’s, and Begg’s tests, though absence of evidence should not be viewed as evidence of absence.
Future direction
As the volume of data on multiportal and uniportal RATS continues to expand, large head-to-head comparative analyses will be critical in solidifying their position as the next evolution of approaches in cardiothoracic surgery. Whilst the outcomes of u-RATS in a general sense are excellent, oncologic parameters, especially lymph node clearance/nodal upstaging rates, histopathological margin clearance, and anesthetics/peri-/postoperative pain results, must be a focus for future studies.
Conclusions
The present systematic review and meta-analysis highlights that the u-RATS approach in expert hands is safe and efficacious, with fast procedural times, short post-operative LOS, and exceptionally low rates of conversion. The primary caveat is that these procedures are predominantly being performed by experts in the field and is the most significant limitation of the study. Additional analyses as these procedures are increasingly utilized, and not necessarily by those considered to be experts in the technique, will be essential in solidifying uniportal robotic surgery as the future standard of care in cardiothoracic surgery.
Acknowledgments
Funding: This research is supported by an Australian Government Research Training Program (RTP) scholarship (ARWS).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coco D, Leanza S. Current perspective on uniportal and multiportal video-assisted thoracic surgery during lobectomy for lung cancer. Kardiochir Torakochirurgia Pol 2022;19:146-51. [Crossref] [PubMed]
- AlShimali H, AlGhunaim E, AlEid Y, et al. Safety and effectiveness of uniportal video-assisted thoracoscopic surgery compared to triportal surgery: a single institution experience. Kardiochir Torakochirurgia Pol 2022;19:240-2. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Institute of Health Economics. Quality Appraisal of Case Series Study Tool. Institute of Health Economics, Edmonton; 2016.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- E H. Hybrid uniportal robotic-assisted thoracoscopic surgery using video-assisted thoracoscopic surgery staplers: technical aspects and results. Ann Cardiothorac Surg 2023;12:34-40. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted sleeve resections: surgical technique and initial experience of 30 cases. Ann Cardiothorac Surg 2023;12:9-22. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Prado RF, Garcia-Perez A, et al. Bilateral uniportal robotic-assisted thoracic surgery sleeve lobectomy for a bilateral endobronchial lung cancer. Ann Cardiothorac Surg 2023;12:64-6. [Crossref] [PubMed]
- Mercadante E, Martucci N, De Luca G, et al. Early experience with uniportal robotic thoracic surgery lobectomy. Front Surg 2022;9:1005860. [Crossref] [PubMed]
- Motas N, Gonzalez-Rivas D, Bosinceanu ML, et al. Uniportal robotic-assisted thoracic surgery pneumonectomy. Ann Cardiothorac Surg 2023;12:67-9. [Crossref] [PubMed]
- Ning Y, Chen Z, Zhang W, et al. Uniportal three-arm robotic-assisted thoracic surgery right upper lobe and carinal sleeve resection. Ann Cardiothorac Surg 2023;12:70-2. [Crossref] [PubMed]
- Paradela M, Garcia-Perez A, Fernandez-Prado R, et al. Uniportal robotic versus thoracoscopic assisted surgery: a propensity score-matched analysis of the initial 100 cases. Ann Cardiothorac Surg 2023;12:23-33. [Crossref] [PubMed]
- Park SY, Lee JH, Kim YH, et al. Multi-institutional surgical outcomes of robotic single-port surgery: a Korean experience. Ann Cardiothorac Surg 2023;12:41-5. [Crossref] [PubMed]
- Ureña A, Déniz C, Muñoz A, et al. Uniportal robotic-assisted thoracoscopic surgery: resection of the first rib. Ann Cardiothorac Surg 2023;12:62-3. [Crossref] [PubMed]
- Vincenzi P, Lo Faso F, Eugeni E, et al. Uniportal robotic-assisted thoracoscopic surgery for early-stage lung cancer with the Da Vinci Xi: Initial experience of two cases. Int J Med Robot 2023;19:e2477. [Crossref] [PubMed]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Manolache V, Gonzalez-Rivas D, Bosinceanu ML, et al. Uniportal robotic-assisted thoracic surgery for mediastinal tumors. Ann Cardiothorac Surg 2023;12:139-41. [Crossref]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted major pulmonary resections. Ann Cardiothorac Surg 2023;12:52-61. [Crossref] [PubMed]
- Dobbs RW, Halgrimson WR, Talamini S, et al. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol 2020;38:897-905. [Crossref] [PubMed]
- Yang B, Chen R, Lin Y, et al. Single-port robotic surgery for mediastinal tumors using the da vinci SP system: Initial experience. Front Surg 2022;9:1043374. [Crossref] [PubMed]
- Wilson-Smith AR, Anning N, Muston B, et al. The learning curve of the robotic-assisted lobectomy-a systematic review and meta-analysis. Ann Cardiothorac Surg 2023;12:1-8. [Crossref] [PubMed]







