Short-term outcomes of uniportal robotic-assisted thoracic surgery anatomic pulmonary resections: experience of Shanghai Pulmonary Hospital
Introduction
Compared with conventional open surgery, minimally invasive lobectomy has been well demonstrated to be associated with reduced post-operative (post-op) complications, hospital stay, surgical pain, and possibly better long-term survival (1,2). Since the first robotic-assisted surgery was performed in 1985 (3), robotic-assisted surgery has been widely accepted and has been applied to a variety of patients across various disciplines. In the field of thoracic surgery, robotic-assisted surgery is most frequently used for major pulmonary resections in patients with pulmonary lesions, such as benign and malignant lung tumors. In most centers, robotic-assisted pulmonary resections have been performed with a multi-portal approach, with usually 4–5 ports; this approach has been well described (4-8). With more advanced technology, progressively more complicated procedures, such as sleeve lobectomies and carinal sleeve resections, have been performed with robotic-assisted surgery (9-12). In this paper, we share our experiences of the uniportal robotic-assisted thoracic surgery (U-RATS). We found that U-RATS was more advantageous than biportal-RATS (B-RATS) in major pulmonary resection. We conducted a retrospective study to compare the short-term outcomes after U-RATS and B-RATS.
Methods
This was a retrospective study which was reviewed and approved by the ethics committee of Shanghai Pulmonary Hospital, Tongji University. All patients signed an informed consent form after personal counseling by an independent research coordinator.
Patients
From March 2021 to June 2022, 109 patients with pulmonary masses or nodules who had undergone robotic assisted anatomic pulmonary resection were recruited. Eligible patients were 18 to 80 years old, with satisfactory preoperative laboratory testing, adequate pulmonary function, and an American Society of Anesthesiologists score of I to III.
Surgery and post-op management
All patients accepted general anesthesia with double-lumen endotracheal intubation. A Da Vinci Xi surgical robot (Intuitive Surgical, Inc., Santa Clara, CA, USA) was used to perform the procedures.
Incision selection
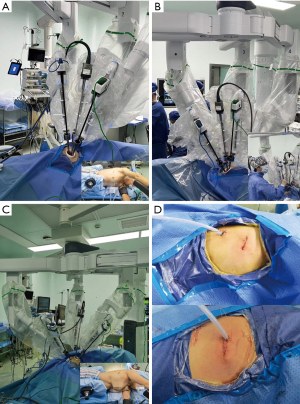
U-RATS was performed though a 4 cm incision in the 4th or 5th intercostal space (ICS) in the mid-axillary line, while B-RATS was performed though a 4 cm incision in the 4th ICS in the anterior-axillary line and a 1.5 cm incision in the 7th ICS in the mid-axillary line (Figure 1A-1C).
Robotic arms selection and arrangement
The combination of the robotic arms were either 1#, 2# and 3#, or 2#, 3# and 4#. The camera port was always in the middle; with 1#, 2# and 3# arms working, the camera port is on the 2# arm and with 2#, 3# and 4# arms working, the camera port is on the 3# arm. To avoid collision, we usually canceled 4# arm on the right side (2# arm for camera) and 1# arm on the left side (3# arm for camera). During U-RATS, the 30-degree camera, left and right arms were all placed in the incision with a protector. This approach is different from the U-RATS procedures which have been previously reported (13). We found that this arrangement is more comfortable for the main surgeon when operating. For the B-RATS procedure, the robotic arm selection and arrangement have been previously published and will hence not be elaborated on (14) (Figure 1A-1C).
Instrument selection and utilization
The instruments used on the right hand included the hook-cautery, curved scissors, Maryland bipolar forceps, ultrasound scalpel or needle holder Suture CutTM. The instruments used on the left hand included the fenestrated bipolar forceps. Additionally, an assistant surgeon used oval forceps (to retract the lung, or suction to optimize the operative view). The assistant placed instruments through the same 3 cm incision (Figure 1B). The instruments for uniportal video-assisted thoracic surgery (U-VATS) and open procedures should always be prepared for urgent situations, as outlined in the recommendations of the pioneering Spanish team. The dissection of vessels, fissures, bronchi and suturing of the anastomosis were performed by the principal surgeon, using the Da Vinci Xi surgical robot system. Since we don’t have the Robotic stapler in our hospital, we use the U-VATS technique, where the endoscopic linear stapler was triggered by the assistant on the operation table. All surgeries were performed by the same surgical group headed by one experienced surgeon (LJ) and an experienced assistant (YN) (Figure 1B). A 28 French chest tube was placed for thoracic drainage (Figure 1D).
Technique for anastomosis of sleeve resections
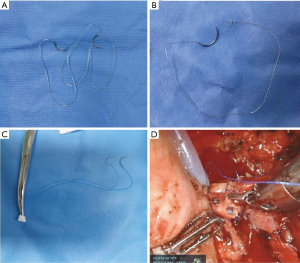
The anastomosis of the bronchus was performed with a suture of Stratafix (SXMD2B402, Spiral PGA-PCL, Tensile Strength Size 3-0, 16 cm × 16 cm, Ethicon Inc.) (Figure 2A). Two kinds of sutures were used. We begin with two single-needle sutures (Figure 2B) which had been described in the published literature (14). We found it more convenient to perform the anastomosis with a double-needle suture. The anastomosis of the pulmonary artery was performed using a half-continuous suture technique with two 5-0 prolene sutures which has also been described in the published literature (14) (Figure 2C,2D).
Post-op management
Enhanced recovery after surgery protocols were routinely applied to every patient as previously reported, including smoking cessation, breathing training, analgesia, early post-op activities, and early post-op extubation (15). All the perioperative short-term outcomes, such as duration of surgery, intra-operative blood loss via thoracic drainage, hospital length of stay and complications, were collected for further statistical analysis. The VAS-scores were taken on the first, third and 30th post-op days.
Statistical analysis
Continuous data were examined for normality with the Shapiro-Wilk test. Data are presented as mean and 95% confidence interval (CI), as either absolute numbers or percentages; statistical significance was set at <0.05. T-test and chi-square test were used for the statistical analysis. All analyses were performed using SPSS software (version 25; SPSS, Chicago, IL, USA) and GraphPad Prism (version 5; San Diego, CA, USA).
Results
Baseline characteristics are demonstrated in Table 1. All surgeries were listed in Table 2. No significant differences were found between these two groups. Perioperative outcomes suggest that the mean duration of surgery of the two groups (U-RATS vs. B-RATS) was 124.1 (106.2–141.8) vs. 103.6 (93.1–114.2) min (P=0.049), mean intraoperative blood loss was 131.7 (86.17–177.3) vs. 143.1 (115.5–170.8) mL, mean post-op hospital stay was 3.83 (3.08–4.56) vs. 3.05 (2.69–3.41) days (P=0.037), and the thoracic drainage of the first day after surgery was 230.9 (164.7–297.0) vs. 207.1 (173.4–240.9) mL, respectively. The VAS-scores showed significant differences in the 1st post-op day [3.83 (3.22–4.43) vs. 4.57 (4.27–4.88), P=0.018] and 3rd post-op day [3.69 (3.25–4.12) vs. 4.363 (4.03–4.68), P=0.026], with no significant difference on the 30th post-op day (Table 3). Seven cases were converted to U-VATS [2 (6.89%) vs. 5 (6.25%)], while 6 cases accepted intraoperative blood transfusion [1 (3.44%) vs. 5 (6.25%)], U-RATS vs. B-RATS respectively. No cases were converted into an open procedure. Short-term outcomes suggest no significant difference in sleeve resections between the subgroups as listed in Table 4. Post-op complications are listed in Table 5. No differences between the two groups were identified. One case suffered from a bronchopleural fistula, which was cured by conservative treatment in one month. No perioperative mortality occurred in either group.
Table 1
| Patient variables of interest | Total (n=109) | U-RATS (n=29) | B-RATS (n=80) | P |
|---|---|---|---|---|
| Age, years | 60.18 (58.05–62.31) | 58.93 (54.73–63.13) | 60.74 (58.23–63.25) | 0.439 |
| Patients ≥70 years | 21 (19.26%) | 6 (20.68%) | 15 (18.75%) | 0.789 |
| Sex (male) | 65 (59.63%) | 19 (65.52%) | 46 (57.75%) | 0.512 |
| BMI (kg/m2) | 24.58 (23.89–25.27) | 24.38 (23.18–25.59) | 25.31 (23.8–25.53) | 0.711 |
| Cardiovascular diseases | ||||
| Hypertension | 44 (40.36%) | 11 (37.93%) | 33 (41.25%) | 0.827 |
| Diabetes mellitus | 37 (33.94%) | 8 (27.58%) | 29 (36.25%) | 0.495 |
| Coronary artery disease | 13 (11.93%) | 4 (13.79%) | 9 (11.25%) | 0.743 |
| Smoking | 54 (49.54%) | 15 (51.72%) | 39 (48.75%) | 0.831 |
| Pulmonary function | ||||
| FEV1 | 2.48 (2.35–2.61) | 2.45 (2.22–2.67) | 2.49 (2.33–2.65) | 0.754 |
| FEV1 (% predicted) | 97.23 (93.65–100.8) | 94.46 (88.22–100.7) | 98.46 (94.02–102.9) | 0.307 |
| DLCO (% predicted) | 110.3 (105.3–115.2) | 104.1 (96.73–111.5) | 113.0 (106.6–119.4) | 0.101 |
| Gas exchange | ||||
| PaO2 (mmHg) | 88.69 (86.98–90.40) | 87.33 (84.67–89.99) | 89.30 (87.09–91.50) | 0.294 |
| PaCO2 (mmHg) | 40.64 (39.93–41.35) | 40.12 (38.95–41.29) | 40.87 (39.97–41.77) | 0.333 |
| SaO2 (%) | 97.30 (97.13–97.47) | 97.27 (96.99–97.54) | 97.31 (97.09–97.53) | 0.807 |
| Size of the lesions (cm) | 2.25 (1.98–2.52) | 2.4 (1.88–2.91) | 2.18 (1.85–2.50) | 0.455 |
| Duration of surgery (min) | 109.1 (99.9–118.2) | 124.1 (106.2–141.8) | 103.6 (93.1–114.2) | 0.049* |
| Intra-op blood loss (mL) | 140.1 (116.8–163.4) | 131.7 (86.17–177.3) | 143.1 (115.5–170.8) | 0.669 |
| Thoracic drainage (1st day) | 213.4 (183.6–243.3) | 230.9 (164.7–297.0) | 207.1 (173.4–240.9) | 0.489 |
| Blood transfusion% | 6 (5.51%) | 1 (3.44%) | 5 (6.25%) | – |
| Conversion (U-VATS) | 7 (6.42%) | 2 (6.89%) | 5 (6.25%) | – |
| Hospital length of stay (days) | 3.25 (2.93–3.58) | 3.83 (3.08–4.56) | 3.05 (2.69–3.41) | 0.037* |
*, P<0.05, compared U-RATS and B-RATS. U-RATS, uniportal robotic-assisted thoracic surgery; B-RATS, biportal-robotic-assisted thoracic surgery; BMI, body mass index; FEV, forced expiratory volume; DLCO, diffusing capacity of the lungs for carbon monoxide; U-VATS, uniportal video-assisted thoracic surgery.
Table 2
| Characteristics of surgeries | Total (n=109) | U-RATS (n=29) | B-RATS (n=80) |
|---|---|---|---|
| Lobectomy | 57 | 10 | 47 |
| Right upper lobe | 10 | 2 | 8 |
| Right middle lobe | 12 | 2 | 10 |
| Right lower lobe | 17 | 4 | 13 |
| Right upper + middle lobe | 2 | 1 | 1 |
| Left upper lobe | 13 | 0 | 13 |
| Left lower lobe | 4 | 1 | 2 |
| Sleeve resection | 28 | 12 | 16 |
| Right upper lobe | 7 | 3 | 4 |
| Right middle lobe | 2 | 1 | 1 |
| Right lower lobe | 4 | 1 | 3 |
| Left upper lobe | 8 | 5 | 3 |
| Lest lower lobe | 5 | 1 | 4 |
| Carinal resection | 2 | 1 | 1 |
| Segmentectomy | 23 | 7 | 16 |
| Pneumonectomy | 1 | 0 | 1 |
RATS, robotic-assisted thoracic surgery; U-RATS, uniportal robotic-assisted thoracic surgery; B-RATS, biportal-robotic-assisted thoracic surgery.
Table 3
| VAS-score | Total (n=109) | U-RATS (n=29) | B-RATS (n=80) | P |
|---|---|---|---|---|
| 1st post-op day | 4.37 (4.09–4.65) | 3.83 (3.22–4.43) | 4.57 (4.27–4.88) | 0.018* |
| 3rd post-op day | 4.18 (3.92–4.45) | 3.69 (3.25–4.12) | 4.363 (4.03–4.68) | 0.026* |
| 30th post-op day | 1.95 (1.71–2.21) | 1.79 (1.32–2.26) | 2.01 (1.72–2.31) | 0.436 |
*, P<0.05, compared U-RATS and B-RATS. VAS, visual analogue scale; post-op, post-operative; U-RATS, uniportal robotic-assisted thoracic surgery; B-RATS, biportal-robotic-assisted thoracic surgery.
Table 4
| Patient variables of interest | Total (n=109) | U-RATS (n=29) | B-RATS (n=80) | P |
|---|---|---|---|---|
| Duration of surgery (min) | 146.7 (130.8–162.6) | 161.0 (144.5–177.5) | 135.9 (110.6–161.2) | 0.111 |
| Intra-op blood loss (mL) | 194.6 (147.9–241.4) | 185.0 (109.9–260.1) | 201.9 (134.9–268.8) | 0.721 |
| Thoracic drainage (1st day) | 388.2 (351.4–425.0) | 422.5 (369.7–475.3) | 362.5 (310.6–414.4) | 0.098 |
| Blood transfusion% | 4 (14.28%) | 1 (8.33%) | 3 (18.75%) | 0.613 |
| Conversion (U-VATS) | 5 (17.85%) | 2 (16.66%) | 3 (18.75) | – |
| Hospital length of stay (days) | 5.53 (5.11–5.96) | 5.91 (5.28–6.55) | 5.25 (4.65–5.85) | 0.115 |
| BPF | 1 (3.57%) | 0 | 1 (6.25%) | – |
*, P<0.05, compared U-RATS and B-RATS. U-RATS, uniportal robotic-assisted thoracic surgery; B-RATS, biportal-robotic-assisted thoracic surgery; U-VATS, uniportal video-assisted thoracic surgery; BPF, bronchopleural fistula.
Table 5
| Postoperative complications | Total (n=109) | U-RATS (n=29) | B-RATS (n=80) |
|---|---|---|---|
| BPF, n (%) | 1 (0.92) | 0 | 1 (3.44) |
| Pulmonary infection, n (%) | 17 (15.59) | 5 (17.24) | 12 (15.00) |
| Re-intubation, n (%) | 1 (0.92) | 0 | 1 (1.25) |
| Atelectasis, n (%) | 15 (13.76) | 4 (13.79) | 11 (13.75) |
U-RATS, uniportal robotic-assisted thoracic surgery; B-RATS, biportal-robotic-assisted thoracic surgery; BPF, bronchopleural fistula.
Discussion
With the advances of robotic surgical technology, robotic-assisted approaches have come to be wildly accepted as viable forms of minimally invasive thoracic surgery (9). Robotic-assisted thoracic surgery has been proven to be feasible and safe, with proponents citing improved instrument control, ergonomics and improved intra-operative views, which is especially helpful for complicated procedures (16,17). Since uni-portal VATS had been wildly accepted for decades (18-20), we attempted to perform uni-portal RATS and succeeded in almost all kinds of major pulmonary resections, from segmentectomies to carinal sleeve resections (21-25). In this paper, we outline the results of our retrospective study examining the short-term outcomes after U-RATS and B-RATS and share our experiences with the U-RATS technology. We practiced our first case of B-RATS lobectomy in March 2021. The first attempt of a lobectomy by U-RATS was achieved in 2022. After that, we launched a clinical trial between U-RATS and B-RATS. Randomization was conducted in the patients with a computer-generated random numbers table. We then attempted complicated procedures with U-RATS, such as sleeve resections and carinal reconstructions. Overall, U-RATS along with B-RATS were deemed safe and feasible techniques for minimally invasive thoracic surgery, even for complicated procedures. Short-term outcomes suggest that the duration of surgery of U-RATS is longer than B-RATS, though this may be caused by an imbalance of more complicated procedures in the U-RATS cohort, with 12/29 (41.37%) sleeve resections in U-RATS group versus only 16/80 (20%) sleeves in B-RATS group. We compared the outcomes of these subgroups and found no significant difference. This also helps explain why the hospital length of stay is a longer in U-RATS group. Five cases of B-RATS group converted to VATS, while two case of U-RATS converted to U-VATS. In our initial experience of the U-RATS procedure, we consider that the technique should be used for rigorously selected cases. Neoadjuvant therapy was not a contraindication for U-RATS, but a huge lesion and dense adhesions of the thoracic cavity were contraindications due to the difficulties in obtaining exposure of important structures. There were no differences in intra-operational blood loss and thoracic drainage at day one post-op in these two groups. This may suggest that U-RATS maintains an advantage for complicated procedures. No major post-op complications occurred in either group. Only one patient in B-RATS suffered from bronchopleural fistula; they underwent a right lower lobe sleeve lobectomy and was cured by conservative treatment. It was the second case of sleeve resection. We initially utilised the single-needle suture, however we found this was not adequate for sleeve resections. A double-needle suture (Stratafix), however, facilitated the bronchial anastomosis far more easily, despite the greater tensile forces on the tissues.
Post-op pain was mainly caused by the trauma of the incision and the thoracic drainage tube. Minimally invasive thoracic surgery relieved post-op pain and reduced the incidence of the post-op complications (26,27). In this study, VAS-scores were found to be significantly lower in U-RATS group (1st and 3rd post-op days). It may be caused by the position of the drainage tube. We found that the pain caused by the drainage tube is pronounced when in the 7th ICS. When the tube was removed, VAS-score showed no difference between two groups. With better relief of post-op pain, U-RATS may be more popular in further clinical work and may have a lower incidence of post-op complications in large sample analyses.
There are limitations to the U-RATS technique. Firstly, as we have stated, the U-RATS technique should only be used in selected cases or it may cause unexpected bleeding, conversion to open procedures or even more critical events. Secondly, the U-RATS procedure should be performed by an experienced VATS surgeon and with an experienced VATS assistant who could cope with emergent situations. Since we had such a rich experience in U-VATS surgery, we could convert to U-VATS to deal with emergent situations. Lastly, since the exposure under U-RATS was more difficult than in B-RATS or multi-portal RATS procedures, hemostasis is quite important for all procedures, especially complicated cases. With regards to the retrospective study design of 29 cases of U-RATS showing only short-term outcomes, the results may suffer from lower reliability and selection bias. A single-center, open-labeled, prospective randomized clinical trial comparing U-RATS and B-RATS for NSCLC is currently being conducted in our center. Long-term outcomes and survival surveillance will be revealed.
Conclusions
In our experiences, both U-RATS and B-RATS were safe and feasible techniques for minimally invasive thoracic surgery in selected cases. There are notable benefits, such as a more convenient robotic arm arrangement, in U-RATS procedures. U-RATS procedures may also lead to a better relief of the post-op pain and may reduce the incidence of post-op complications. More rigorous data in the way of a randomized, prospective control trial will further delineate the benefits of these approaches.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev 2013;22:382-404. [Crossref] [PubMed]
- Demmy TL, Yendamuri S, D'Amico TA, et al. Oncologic Equivalence of Minimally Invasive Lobectomy: The Scientific and Practical Arguments. Ann Thorac Surg 2018;106:609-17. [Crossref] [PubMed]
- Ponnusamy K, Mohr C, Curet MJ. Clinical outcomes with robotic surgery. Curr Probl Surg 2011;48:577-656. [Crossref] [PubMed]
- Brooks P. Robotic-Assisted Thoracic Surgery for Early-Stage Lung Cancer: A Review. AORN J 2015;102:40-9. [Crossref] [PubMed]
- Watkins AA, Quadri SM, Servais EL. Robotic-Assisted Complex Pulmonary Resection: Sleeve Lobectomy for Cancer. Innovations (Phila) 2021;16:132-5. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin 2014;24:135-41. v. [Crossref] [PubMed]
- Qiu T, Zhao Y, Xuan Y, et al. Robotic sleeve lobectomy for centrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. J Thorac Cardiovasc Surg 2020;160:838-46.e2. [Crossref] [PubMed]
- Hu D, Wang Z, Tantai J, et al. Robotic-assisted thoracoscopic resection and reconstruction of the carina. Interact Cardiovasc Thorac Surg 2020;31:912-4. [Crossref] [PubMed]
- Qiu T, Zhao Y, Xuan Y, et al. Robotic-assisted double-sleeve lobectomy. J Thorac Dis 2017;9:E21-5. [Crossref] [PubMed]
- Cohen BD, Marshall MB. Robotic-assisted tracheobronchial surgery. J Thorac Dis 2020;12:6173-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. [Crossref] [PubMed]
- Qu JC, Zhang WT, Jiang L. Two-port robotic sleeve lobectomy using Stratafix sutures for central lung tumors. Thorac Cancer 2022;13:1457-62. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Salati M, Rocco G. The uni-portal video-assisted thoracic surgery: achievements and potentials. J Thorac Dis 2014;6:S618-22. [PubMed]
- Qu R, Hao Z, Zhang Y, et al. Single-center experience of simultaneous bilateral uni-portal video-assisted thoracoscopic surgery for multiple ground-glass opacities. J Cardiothorac Surg 2020;15:69. [Crossref] [PubMed]
- Li Z, Zhao Y, Hu X, et al. Is uni-portal video-assisted thoracic surgery a feasible approach for the surgical treatment of bronchopulmonary sequestration? J Thorac Dis 2020;12:414-21. [Crossref] [PubMed]
- Abu Akar F, Chen Z, Yang C, et al. Enhanced recovery pathways in thoracic surgery: the Shanghai experience. J Thorac Dis 2018;10:S578-82. [Crossref] [PubMed]
- Cai J, Song N, Jiang L. Left sleeve pneumonectomy via uniportal video-assisted thoracoscopic approach. Thorac Cancer 2022;13:506-9. [Crossref] [PubMed]
- Jiang L, Bao Y, Liu M, et al. Uniportal video-assisted thoracoscopic left basilar segmentectomy. J Thorac Dis 2014;6:1834-6. [PubMed]
- Jiang L, Wu L, Roque SR, et al. A novel tourniquet technique for transient pulmonary artery occlusion during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2018;156:816-8. [Crossref] [PubMed]
- González-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic sleeve resections: Where are the limits? JTCVS Tech 2020;2:160-4. [Crossref] [PubMed]
- Fan CJ, Chien HL, Weiss MJ, et al. Minimally invasive versus open surgery in the Medicare population: a comparison of post-operative and economic outcomes. Surg Endosc 2018;32:3874-80. [Crossref] [PubMed]
- Biere SS, Maas KW, Bonavina L, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011;11:2. [Crossref] [PubMed]






