The learning curve of the robotic-assisted lobectomy—a systematic review and meta-analysis
Introduction
Since its mainstream inception in the early 2000s, robotic surgery has come to be well-established in most surgical subspecialties (1). Robotic-assisted thoracic surgery (RATS), specifically the robotic-assisted lobectomy, has proven itself to be safe, oncologically efficacious and economically cost-effective, offering patients the potential for improved cosmesis, shorter intensive care and hospital length-of-stays, with equivalent or better outcomes compared to traditional approaches (2). Encouraging short-term results have been reiterated in the preliminary outcomes of the Robotic-Assisted Versus Video-Assisted Thoracoscopic Lobectomy (RVlob) trial (3). The significant learning curve of robotic lobectomy, however, continues to be presented as a limiting factor to its ongoing uptake, with the overwhelming volume of these surgeries being performed in centers of excellence where strong experience in minimal access approaches (e.g., video-assisted thoracoscopic surgery) is the baseline. However, no exact, holistic quantification of the learning curve of this robotic approach has been made to date, begging the question as to whether this is an outdated assumption, versus fact. This systematic review sort to clarify the learning curve for robotic-assisted lobectomy based on the existing literature.
Methods
Literature search strategy
The methods for this systematic review adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) updated statement (4). Four electronic databases were used to perform the literature searches, encompassing EMBASE, Ovid MEDLINE, PubMed and SCOPUS. These databases were searched from the date of database inception through to 24th July 2022. For examination of the learning curve associated with robotic lobectomy, a search strategy using the combination of keywords and Medical Subject Headings (MeSH) including (robotic assisted OR robotic surgery OR robotic thoracic surgery OR RATS OR RAT OR RTS) AND (lobar resection OR lobectomy) AND (learning curve) was utilized (see Figure S1). Predefined selection criteria were applied to assess for inclusion. Each study was screened independently by two co-authors, with any conflicts resolved prior to progression. Where the title and/or abstract provided insufficient detail in the determination of relevance for additional screening, a full-text review of the record was carried out in the first instance. Reference lists of the included studies were reviewed at completion of the database search to identify any extra, relevant studies not already included.
Inclusion and exclusion criteria
Studies were included in the review if they examined the learning curve of their operators undertaking robotic lobectomy; specifically, reported perioperative outcomes across time. Studies were excluded for: (I) non-English reporting; (II) case reports/small case series with <10 subjects; (III) registries without recruiting details; (IV) no mention of operator learning curve.
Primary and secondary endpoints
The primary endpoint for analysis was operator learning curve results, where clear distinction of incremental or decremental outcomes across case-volume and time were reported (i.e., in distinct operating phases with an overall aggregated case volume, numeric patient clusters, cumulative sum charts, linear regression, etc.). Secondary endpoints of interest included mean console and dock time, blood loss, chest tube duration, length of hospital stay and total lymph nodes removed (with stations if available).
Data extraction, critical appraisal and quality assessment
Two independent reviewers extracted data directly from publication texts, tables and figures. A third reviewer independently reviewed and confirmed all extracted data. Differing opinions between the two main reviewers were resolved through discussion led by the primary investigator. Attempts were made to clarify insufficient/indistinct data from authors of included studies, as required. Data was extracted in a way that each study was effectively treated as a case series, irrespective of underlying design. Outcomes of interest that were not reported (or were not reported sufficiently for meta-analysis) were namely: renal impairment, hypertension, diabetes, chronic obstructive pulmonary disease, atrial fibrillation, peripheral vascular disease, coronary artery disease, prior acute coronary syndrome or myocardial infarction, cerebrovascular accident or transient ischemic attack, reoperation and readmission (30-day). The Canadian Institute of Health Economics Quality Appraisal score was used as the quality assessment tool (5). Studies were defined as low quality with scores <10/19, moderate quality with score 11–15/19 and high quality with scores >15/19 (see Table S1). Risk of bias was assessed using the “Risk of Bias in Non-randomized Studies of Interventions” (ROBINS-I) tool and is visually presented (see Figure S2) (6).
Statistics
A meta-analysis of proportions or means was performed for categorical and continuous variables, as appropriate, by an independent reviewer. A random effects model was used to account for differing regions, surgeon experience, surgical technique and equipment, and management protocols across the included studies. Means and standard deviations (SDs) were calculated from the median, where reported, using the methods described by Wan and colleagues (7). Pooled data and SD were presented as N (%) ± SD with 95% confidence intervals (CIs). For outcome data, heterogeneity amongst studies was assessed using the I2 statistic. Thresholds for these values considered as low, moderate and high heterogeneity were 0–49%, 50–74% and greater than or equal to 75%, respectively. Meta-analysis of proportions or means was performed using Stata (version 17.0, StataCorp., Texas, USA). Survival data was calculated from the aggregation of Kaplan-Meier (KM) curves from the included studies, if reported, by utilizing the methods of Guyot and colleagues (8).
Results
Study characteristics
A total of 209 studies on the learning curve of robotic lobectomies were identified in the literature search, with twenty-two progressing to inclusion. The studies included in this review were overwhelmingly retrospective (9-28) versus prospective (29,30) in nature. The majority of studies (twelve) were drawn from North America, with the remainder from Europe (three), China (three), Japan (two) Korea (one) and the Middle East (one). Upon quality assessment, twelve studies were deemed to be of high quality (9,13,15-17,19-22,25,26,29), nine of medium quality (10,11,14,18,23,24,27,28,30) and one of low quality (12). Risk of bias assessment illustrated low-risk in the majority of studies, with only four demonstrating minor concerns (11,23,28,30) (see Figure S2).
Baseline demographic characteristics, operative details and post-operative outcomes
Baseline demographic characteristics and operative details are reported in Table 1. A total of 3,246 patients (30% male) receiving RATS were identified across an operative period of 2011 to 2020. Long-term follow-up data were poorly reported, and hence KM analysis was not carried out. The robotic-assisted lobectomies in this analysis were performed in the established complete port robotic lobectomy (CPRL) robotic-assisted lobectomy-4 (RAL-4) manner. The Da VinciR Surgical System (Intuitive Surgical Inc., Sunnyvale, California, USA) Xi or Si was the primary systems of choice. All studies reported port access sites, anesthetic delivery and ventilation methods. Variably, a combination of working ports, typically 2–3×8 mm and 1–2×12 mm utility ports were utilized, per institution and surgeon preference. The mean age of the cohort was 65.3±5.0 years. FEV1 preoperatively was 2.5±0.2 L/s. Tumor size was 2.2±0.55 cm. Mean operative, console, and dock time was 190.5±53.8, 125.8±33.9, and 10.2±4.0 minutes, respectively. Blood loss was 90.0±85.4 mL. Chest tube duration was 3.5±0.8 days. Lymph node dissection was 14.7±5.0 nodes per case. Length of hospital stay was 6.1±4.6 days. Outcomes of interest including affected lobes, tumor histology and individual staging are reported in supplementary materials (available on request).
Table 1
| Variable of interest | Values identified | 95% confidence interval |
|---|---|---|
| Cohort size (n) | 3,246 | – |
| Males (n) | 972 | – |
| Mean age of cohort (years), mean ± SD | 65.3±5.0 | 63.8–66.8 |
| Mean FEV1 pre-op (L/s), mean ± SD | 2.5±0.2 | 2.5–2.3 |
| Mean tumor size (cm), mean ± SD | 2.2±0.55 | 2.2–2.3 |
| Mean operation time (min), mean ± SD | 190.5±53.8 | 99.7–204.3 |
| Mean console time (min), mean ± SD | 125.8±33.9 | 58.3–155.5 |
| Mean dock time (min), mean ± SD | 10.2±4.0 | 10.7–13.6 |
| Mean blood loss (mL), mean ± SD | 90.0±85.4 | 28.6–111.3 |
| Mean chest tube time (days), mean ± SD | 3.5±0.8 | 2.5–4.0 |
| Mean length of stay (days), mean ± SD | 6.1±4.6 | 4.8–6.4 |
| Mean lymph nodes removed (nodes), mean ± SD | 14.7±5.0 | 12.3–16.8 |
| Mean conversion to open procedure (n), mean ± SD | 2.8±2.6 | – |
| Mean learning curve (case number), mean ± SD | 25.3±12.6 | 14–56 |
SD, standard deviation; FEV1, forced expiratory volume in 1 second; pre-op, pre-operative.
Learning curve outcomes
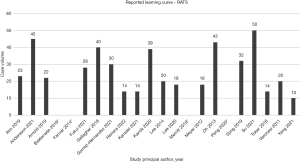
Learning curve outcomes were reported via cumulative sum chart (CUSUM) analysis with or without risk adjustment, non-CUSUM linear regression modelling or outcome-specific analysis. These values were aggregated using meta-analysis with a random effects model. Case volume for baseline proficiency was 25.3±12.6 cases. All included studies reported the primary endpoint initially sought. Sensitivity analysis of high-quality studies did not significantly alter the case volume required for proficiency, with 24.8±3.6 cases required. Figure 1 details the reported learning curve volumes required for technical proficiency across the included studies.
Discussion
Robotic-assisted approaches in thoracic surgery are growing in popularity, all in an effort to offer patients equivalent or improved outcomes in comparison to more invasive, traumatic techniques (1). However, due to the often-cited steep learning curve associated with these approaches and the higher initial costs, RATS remains localized to centers of excellence and largely limited to surgeons proficient with video-assisted thoracoscopic (VATS) approaches. Alongside this steep learning curve, it has also been questioned as to whether it represents a pragmatic modality when outcomes with VATS as a minimally invasive approach are excellent. The aim of this systematic review and meta-analysis was to provide the most up-to-date, comprehensive assessment of the literature examining the learning curve of robotic-assisted lobectomy. No systematic review or meta-analysis has previously been conducted on this subject, with only one recent review paper outlining coherent, summarized evidence to date (31).
Reporting of learning curve experience across included studies
Depending on the metric of interest and the degree of initial technical competency with VATS, the learning curve for robotic-assisted lobectomy was identified to be 25.3±12.6 cases. These values encompass acceptable case volumes for subjective surgeon comfort and ease with the robotic system, reflect the point of reduction of operative times towards the ‘plateau’ and ‘mastery’ phases of learning, and complication rates such as postoperative air leaks and transfusion amounts (10). Unsurprisingly, in those cohorts where surgeons were already considered experts in VATS (without additional quantification of degree of expertise), or when they were the sole robotic operators in an institution, technical proficiency was achieved earlier in the learning process (22,23,27). This is likely due to the operator familiarity with adjusting for heavily magnified views, ease of navigation around obstructions, repetition learning and a focus on minimizing bleeding given the disproportionate effect of small amounts of bleeding on visualization in both VATS and particularly in RATS. Accordingly, in these institutions, there did not appear to be any statistical difference in complication rates in those studies with comparison to VATS (14,25). RATS’ safety profile does appear to be preserved even in those cohorts where operators do not have significant previous VATS experience (20).
It is difficult to parameterize an acceptable definition of what constitutes a ‘learning curve’, given the inherent variability of inter-operator experience, previous exposure to minimally invasive techniques, and that proctorship and institutional access to minimal access surgery were not elaborated upon or discussed within the literature, bar superficial comments on prior experience or mandatory clinical requirements. The papers included for analysis, indeed representing the most appropriate pool with relevant data on the learning curve of robotic lobectomies, are notably technical papers at their core; more relevant information for a surgical audience, such as the prior number of VATS cases done per surgeon, experience on earlier robotic platforms, proctorship and so on, are unfortunately not discussed in significant detail. Only three studies made specific, though limited, mention of proctorship (19,20,24), with two studies reporting mandatory proctorship cases required by their hospital’s licensing board, and one study outlining a recommendation for proctorship after the initial learning phase.
With respect to the variability of case volumes, the highest case volume required to achieve technical competency was noted in the cohort described by Su et al. (10). This was largely attributed to the cases being performed by four separate surgeons with significant variability in experience, with the most senior surgeon having fifteen years of VATS experience, a mid-career surgeon with eight years of experience, and two immediate post-fellowship surgeons. None of the operators had any prior experience with robotic procedures. Of note, independent of this prior VATS experience, their case volumes required to achieve competency was consistent across all operators–a finding suggestive of a specific learning curve for RATS. The lowest case volume required to achieve technical competency was noted in Yang et al.’s cohort (29), with only ten cases suggested to overcome the learning curve. A strong suggestion was made, however, that mastery of the technique would not be achieved until far later with over fifty cases. Docking times were higher in some phases of their learning curve, where the attending surgeon docked the arms in the earlier phases with subsequent transition to the fellows in later phases. Additionally, their teams were also involved in other robotic procedures (i.e., mediastinal resections, esophagectomies), which would also have contributed to more rapid familiarity with the Da Vinci platform.
RATS versus VATS—a significant evolution?
One clear advantage of the robotic approach is the proposed increased clearance of node stations, overall nodal number dissected and reduced nodal upstaging compared to VATS, an important consideration in both early and more advanced cases (20,25,26). Merritt et al. reported a significant increase in total nodes harvested in their robotic cohort versus thoracoscopic patients with 14.21±6.45 versus 10.39±5.68 nodes removed, respectively (25), though other studies reporting this benefit were not able to meet statistical significance; Gallagher makes note that no difference between their VATS and RATS cohort with respect to nodal upstaging was found, however, suggesting that even in the early phases of RATS experience, it can replicate the resection completeness of other approaches even if total nodes removed were not greater in comparison (20). Whether these data are preserved when accounting for operator skill level has yet to be determined given the lack of operator skill data. It is also difficult to ascertain whether the robotic lobectomy confers a benefit in terms of ICU and hospital length of stay in comparison to experienced VATS operators, with some studies reporting no difference between the two approaches (23). The upcoming RVlob randomized control trial will hopefully elucidate the oncologic efficacy and course of recovery in these patients past the short-term (3).
Limitations
There are a number of limitations that need to be considered when examining the results of this review. The principal concern is that the generalizability of the results to those with little training in minimally invasive approaches is limited; surgeons with baseline to minimal levels of competency with VATS will likely face a steeper learning curve than their colleagues who are considered ‘masters’ at this approach who then go on to train in robotics, and as such, case volumes to gain proficiency will vary. The majority of studies in the literature selected for surgeons with good background experience in VATS, assuming they would be the best operators to transition across to robotic approaches, but no clear definition of what constituted acceptable prior experience was provided. Aggregation of specific learning curve outcomes (i.e., operative time reductions) was also not possible given the different metrics investigators utilized.
CUSUM analysis was utilized for five studies (13,15,18,21,29), whereas other studies report trendline regression (similar in principle to CUSUM) (9) or plateaus in improvement across specific outcomes (10-12,19). To the author’s knowledge, there are no means of aggregating these statistical methods, other than through accepting their individual constraints, and aggregating them as a whole. Additionally, analysis of single and multi-surgeon outcomes was aggregated, introducing a degree of inter-rater variability. However, stratifying for single versus multi-surgeon outcomes would too heavily restrict case volumes so as to render aggregation inappropriate. Complexity of pathology was not homogenized across consecutive cases, which is reflected in a bimodal distribution and/or plateauing of operating time in several studies (20,28). The overwhelming majority of the studies included were also retrospective in nature, with the inherent risks of biases entailed in such a study design. This was assessed to as pragmatic a degree as possible.
Conclusions
The robotic-assisted lobectomy has been illustrated to have a reasonable learning curve profile based on the existing literature. For those operators with prior experience in minimally invasive surgery, it is likely that the learning curve will be more forgiving as opposed to a novice, though this is not as critical as is thought. Current evidence on the oncologic efficacy and purported benefits of the robotic approach, including reducing inpatient stay and rates of complication, will be bolstered by upcoming randomized trial results and will be critical in confirming its role as a needed approach in the management of thoracic malignancies.
Acknowledgments
Funding: This research is supported by an Australian Government Research Training Program (RTP) scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soomro NA, Hashimoto DA, Porteous AJ, et al. Systematic review of learning curves in robot-assisted surgery. BJS Open 2020;4:27-44. [Crossref] [PubMed]
- Kim MP. Robotic lobectomy leads to excellent survival in lung cancer patients. J Thorac Dis 2018;10:S3184-5. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Institute of Health Economics. Quality Appraisal of Case Series Study Tool. Institute of Health Economics, Edmonton, 2016.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Su L, Ho H, Stock CT, et al. Surgeon Experience Is Associated With Prolonged Air Leak After Robotic-assisted Pulmonary Lobectomy. Ann Thorac Surg 2022;114:434-41. [Crossref] [PubMed]
- Oh DS, Cho I, Karamian B, et al. Early adoption of robotic pulmonary lobectomy: feasibility and initial outcomes. Am Surg 2013;79:1075-80.
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Song G, Sun X, Miao S, et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis 2019;11:2431-7. [Crossref] [PubMed]
- Peng M, Wang X, Chen C, et al. Report on 153 sequential three-incision robotic-assisted pulmonary resections by a single surgeon: technical details and learning curve. J Thorac Dis 2020;12:741-8. [Crossref] [PubMed]
- Andersson SE, Ilonen IK, Pälli OH, et al. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat Res Commun 2021;27:100362. [Crossref] [PubMed]
- Ahn S, Jeong JY, Kim HW, et al. Robotic lobectomy for lung cancer: initial experience of a single institution in Korea. Ann Cardiothorac Surg 2019;8:226-32. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Bhatnagar V, et al. Defining the learning curve in robot-assisted thoracoscopic lobectomy. Surgery 2019;165:450-4. [Crossref] [PubMed]
- Gómez Hernández MT, Fuentes Gago M, Novoa Valentín N, et al. Robotic anatomical lung resections: Analysis of the learning curve. Cir Esp 2021;99:421-7. (Engl Ed). [Crossref] [PubMed]
- Baldonado JJAR, Amaral M, Garrett J, et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robot Surg 2019;13:663-9. [Crossref] [PubMed]
- Gallagher SP, Abolhoda A, Kirkpatrick VE, et al. Learning Curve of Robotic Lobectomy for Early-Stage Non-Small Cell Lung Cancer by a Thoracic Surgeon Adept in Open Lobectomy. Innovations (Phila) 2018;13:321-7. [Crossref] [PubMed]
- Kanzaki M, Mitsuboshi S, Koen A, et al. Effects of robot- and video-assisted thoracoscopic lobectomy experiences on the learning curve of lobectomy. Turk Gogus Kalp Damar Cerrahisi Derg 2021;29:527-35. [Crossref] [PubMed]
- Fukui T, Kawaguchi K, Tsubouchi H, et al. <Editors’ Choice> Learning curve of robotic lobectomy for lung malignancies by certified thoracic surgeons. Nagoya J Med Sci 2021;83:227-37. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Herrera L, Escalon J, Johnston M, et al. Development of a robot-assisted thoracic surgery (RATS) program. Lessons learned after 2500 cases. J Robot Surg 2022; Epub ahead of print. [Crossref]
- Merritt RE, Kneuertz PJ, D’Souza DM. Successful Transition to Robotic-Assisted Lobectomy With Previous Proficiency in Thoracoscopic Lobectomy. Innovations (Phila) 2019;14:263-71. [Crossref] [PubMed]
- Lee EC, Lazzaro RS, Glassman LR, et al. Switching from Thoracoscopic to Robotic Platform for Lobectomy: Report of Learning Curve and Outcome. Innovations (Phila) 2020;15:235-42. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Karnik N, Yang X, Goussous N, et al. A community hospital’s experience with robotic thoracic surgery. Indian J Thorac Cardiovasc Surg 2020;36:142-7. [Crossref] [PubMed]
- Yang MZ, Lai RC, Abbas AE, et al. Learning curve of robotic portal lobectomy for pulmonary neoplasms: A prospective observational study. Thorac Cancer 2021;12:1431-40. [Crossref] [PubMed]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]
- Paladini P, Meniconi F, Ghisalberti M, et al. Review of the learning curve of video-assisted thoracic surgery & robotic-assisted thoracic surgery lobectomies—similarities and differences. Curr Chall Thorac Surg 2021;3:16.






