Robotic and endoscopic mitral valve repair for degenerative disease
Introduction
Surgical mitral valve repair remains the gold standard for the treatment of degenerative mitral valve disease according to the most recent American Heart Association (AHA) and European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines (1). In 2003, Casselman et al. demonstrated that mitral valve repair could be done safely and effectively using a classic endoscopic approach through a right mini-thoracotomy (2). Classic endoscopic repair shortens hospital and intensive care unit (ICU) stay compared to open sternotomy, and also confers a lower risk of postoperative bleeding, a shorter time to extubation, fewer transfusions, and less postoperative pain (3,4). Classic endoscopic techniques have been shown to be comparable in quality and durability compared to conventional sternotomy. Transthoracic aortic clamping and endo-balloon aortic occlusion both with antegrade and retrograde cardioplegia, along with hypothermic fibrillatory arrest, are all acceptable strategies for classic endoscopic mitral surgery (3,4).
The first robotic endoscopic mitral valve repair was performed by Carpentier in 1998 using an early prototype of the da VinciTM Surgical System, and in 2002 the Food and Drug Administration (FDA) approved the da VinciTM platform for mitral valve surgery in the United States (5). While a robotic endoscopic approach mitral repair offers enhanced visualization of the valve, smaller incisions, and potentially faster recovery, it also mandates more investment, higher costs, greater teamwork, and specialized training (6). The advantages and disadvantages of robotic endoscopic surgery over classic endoscopic repair have not yet been elucidated. Research into the safety of robotic endoscopic mitral valve surgery compared to other minimally invasive approaches has focused on heterogeneous patient cohorts with a wide range of degenerative disease (7-10). We here compare early and late clinical and echocardiographic outcomes across matched patients undergoing classic endoscopic and robotic endoscopic repair.
Methods
From 2011 to 2020, 786 patients underwent minimally invasive mitral surgery, from which we were able to generate 62 matched pairs, totalling 124 patients. Statistical analysis was performed using STATA 13.0 software (StataCorp LP, College Station, TX, USA). Variables are listed as mean ± standard deviation (SD) or median and interquartile range (IQR) according to distribution. Descriptive analysis was performed using Student’s t-tests for continuous variables, and χ2 tests for categoric variables. Significance was defined as a P value of less than 0.05.
Patient characteristics
In this matched analysis, 62 patients underwent minimally invasive cardiac operations via the right mini-thoracotomy classic endoscopic approach, and 62 patients underwent a robotic endoscopic assisted mitral valve repair (Table 1). There was no observed gender difference across either cohort. There was no difference in the classic endoscopic or robotic endoscopic surgery group with respect to age, incidence of New York Heart Association (NYHA) >2 heart failure, diabetes, hypertension, atrial fibrillation, or coronary artery disease. No patients had undergone prior open cardiac surgical intervention. One patient with end-stage renal disease on hemodialysis underwent a classic endoscopic procedure. Preoperative echocardiographic data were comparable between the two groups: left ventricular ejection fraction was 58.00%±8.15% in the robotic endoscopic group and 57.79%±9.29% in the classic endoscopic group. Left ventricular end-diastolic diameter was 5.71±1.11 mm in the robotic endoscopic group and 5.79±0.75 mm in the classic endoscopic cohort. All patients had symptomatic moderate-severe or severe mitral insufficiency of a degenerative nature.
Table 1
| Demographics and comorbidities | All (n=124) | Robotic endoscopic (n=62) | Classic endoscopic (n=62) |
|---|---|---|---|
| Age (years), mean ± SD | 56.1±11.0 | 56.7±10.4 | 55.5±11.6 |
| Female, n (%) | 42 (33.9) | 20 (32.3) | 22 (35.5) |
| NYHA Class III or IV, n (%) | 12 (9.7) | 5 (8.1) | 7 (11.3) |
| Left ventricular ejection fraction (%), mean ± SD | 57.88±8.73 | 57.96±8.15 | 57.79±9.29 |
| Diabetes mellitus, n (%) | 6 (4.8) | 3 (4.8) | 3 (4.8) |
| Dialysis, n (%) | 1 (0.8) | 0 (0.8) | 1 (1.6) |
SD, standard deviation; NYHA, New York Heart Association.
Operative technique
Seven surgeons within our institution performed classic endoscopic and three performed robotic endoscopic procedures. Principal operative techniques did not differ across the two groups. All operations were performed with peripheral bicaval cannulation and either endoballoon aortic occlusion or Chitwood aortic cross-clamping. Intermittent antegrade Del Nido cardioplegia was used and dosed every 60 min. All patients received corrective annuloplasty at the time of surgery, according to surgeon preference. Seventeen percent of patients in the robotic endoscopic cohort underwent full annuloplasty compared with 31% of patients in the classic endoscopic group. Four patients in the robotic endoscopic group underwent edge-to-edge repair compared with five patients in the classic endoscopic group (Table 2). Artificial neo-chordae were used in 30% of patients in the classic endoscopic group and 23% of patients in the robotic endoscopic group. There were no conversions to open sternotomy in this cohort.
Table 2
| Principal techniques | All patients (n=124), n (%) | Robotic endoscopic (n=62), n (%) | Classic endoscopic (n=62), n (%) |
|---|---|---|---|
| Corrective annuloplasty | 124 (100.0) | 62 (100.0) | 62 (100.0) |
| Full annuloplasty | 30 (24.2) | 11 (17.7) | 19 (30.6) |
| Partial annuloplasty | 74 (59.7) | 30 (48.4) | 34 (54.8) |
| Leaflet resection | 61 (49.2) | 30 (48.4) | 31 (50.0) |
| Edge-to-edge repair | 9 (7.3) | 4 (6.5) | 5 (8.1) |
| Artificial chordae | 33 (26.6) | 14 (22.6) | 19 (30.6) |
Matching process
To control for treatment effect, propensity scores were calculated using a nonparsimonious logistic regression model based on eleven patient risk factors (age, sex, non-white race, hypertension, diabetes mellitus (DM), hyperlipidemia, body mass index, preoperative glomerular filtration rate (GFR), preoperative hematocrit, history of liver disease, and concomitant coronary artery bypass grafting (CABG). We performed 1:1 nearest-neighbor matching without replacement with a narrow caliper of 0.01 based on whether patients underwent classic endoscopic mitral valve repair versus robotic endoscopic mitral valve repair. Among matched cohorts, the degree of imbalance for each included variable was evaluated by the absolute standardized difference of means. Outcomes were then compared between the two matched populations.
Kaplan-Meier survival analysis was used to compare freedom from mortality to 10 years among matched classic endoscopic and robotic endoscopic mitral valve repair cohorts. Kaplan-Meier curves were also constructed for freedom from moderate or severe mitral insufficiency at latest follow-up. Histograms of cardiopulmonary bypass (CPB) and aortic cross-clamp times were constructed, and mean bypass and cross-clamp times were compared between classic endoscopic and robotic endoscopic cohorts using Student’s t-tests.
Results
Operative outcomes
There was no difference in early or late mortality at 10 years in either cohort (Figure 1). Patients undergoing robotic endoscopic mitral repair had a significantly longer CPB time when compared to the classic endoscopic cohort, with 148 min of CPB in the robotic endoscopic cohort compared to 133 min in the classic endoscopic group, P=0.03 (Figure 2). Cross clamp time was not statistically significant between robotic endoscopic and classic endoscopic groups, 148±37 and 133±42 min, respectively. Additionally, post-operative length of stay was not statistically significant between the robotic endoscopic and classic endoscopic groups, 6.3±0.5 and 6.0±0.3 days, respectively (Figure 3). No patients in either cohort developed renal failure or wound infection. The classic endoscopic group had a slightly higher risk of prolonged ventilation when compared to the robotic endoscopic group, with three classic endoscopic patients remaining intubated >8 hours post-operatively compared to a single patient in the robotic endoscopic group. There were no unplanned reoperations in either group for rebleeding. Rates of postoperative stroke were comparable between groups (three strokes in the classic endoscopic cohort, and two strokes in the robotic endoscopic cohort; Table 3).
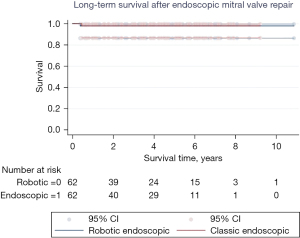
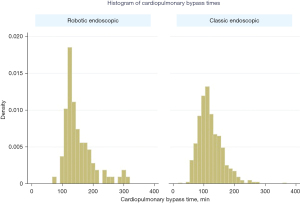
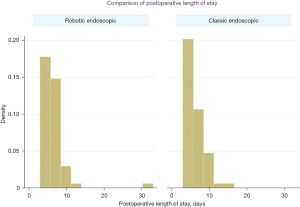
Table 3
| Short term outcomes | All patients (n=124), n (%) | Robotic endoscopic (n=62), n (%) | Classic endoscopic (n=62), n (%) |
|---|---|---|---|
| Any cardiac operation | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal failure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deep sternal infection | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prolonged ventilation or intubation | 4 (3.2) | 1 (1.6) | 3 (4.8) |
| Stroke | 5 (4.0) | 2 (3.2) | 3 (4.8) |
Freedom from moderate or severe mitral regurgitation or mitral valve replacement at last echocardiogram was 86.4% vs. 73.5% at 10 years in the classic endoscopic and robotic endoscopic groups, respectively (P=0.97; Figures 4,5). For the robotic endoscopic group, freedom from mitral regurgitation at 1, 3, and 5 years was 96.3%, 86.4%, and 86.4%, respectively. For the classic endoscopic group, freedom from mitral regurgitation was 100%, 95.2%, and 73.5% at 1, 3, and 5 years, respectively. Overall, the differences between the cohorts were not significantly different. There were three unplanned mitral valve replacements following mitral valve repair in total, two in the classic endoscopic cohort and one in the robotic endoscopic cohort (Table 4). There were five patients who underwent reoperation after their index mitral valve repair, of which three were in the classic endoscopic cohort and two were in the robotic endoscopic cohort. The interval in between operations ranged from 0.1 to 4.4 years. Of the five patients who underwent reoperation, two underwent successful re-repair.
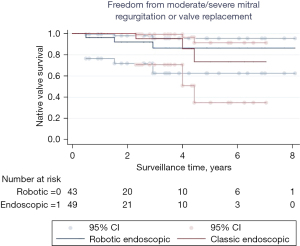
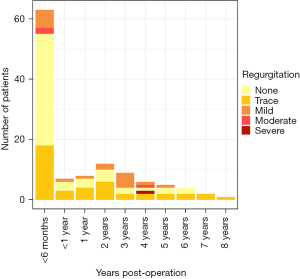
Table 4
| Patient age (years) | Operation type | Interval between operations (years) | Mode of failure after initial repair | Reoperation | Reason for reoperation |
|---|---|---|---|---|---|
| 64 | Classic endoscopic | 4.4 | Progression of original disease | Tissue valve replacement #29 | Ruptured chords to posterior medial commissure and anterior leaflet |
| 66 | Classic endoscopic | 2.3 | Technical error | Mitral valve repair | Override of P2 at site of previous P2 resection and plication |
| 58 | Robotic endoscopic | 1.3 | Technical error | Mechanical valve replacement #31 | Deformed annuloplasty |
| 60 | Robotic endoscopic | 0.1 | Technical error | Mitral valve repair | Small defect around ring at A1 as well as P3 |
| 57 | Classic endoscopic | 0.1 | Technical error | Tissue valve replacement #33 | Dehiscence of neochords |
Discussion
In this study, we show that classic endoscopic and robotic endoscopic mitral valve repair can be performed with comparable outcomes. While bypass times were higher in the robotic endoscopic group, this did not translate to poorer clinical outcomes. One in five isolated primary mitral valve operations in North America is performed using minimally invasive techniques, while only 8% of mitral valve operations are performed via robotic endoscopic mitral surgery (11). The unadjusted mortality rate for less-invasive mitral valve operations such as classic endoscopic and robotic endoscopic, as extracted from the Society of Thoracic Surgeons (STS) database in 2010, is 1.27%. The permanent stroke rate for this same cohort was 1.87% (12). Centers of excellence are best equipped to maximize the rate and durability of mitral valve repair (13). Several groups have reported success with minimally invasive mitral valve surgery and previous research has demonstrated similar functional outcomes compared to a traditional sternotomy approach (14,15). Asymptomatic patients without pulmonary hypertension, left ventricular dilatation, or dysfunction have excellent postoperative outcomes. Our group has previously shown that even patients at higher risk, such as those with pulmonary hypertension, can undergo classic endoscopic operations safely (16). The extent to which clinical outcomes may differ between classic endoscopic and robotic endoscopic mitral repair has not been fully described.
Differences in mitral valve repair technique in this series are probably related to surgeon preference, as some surgeons who prefer partial annuloplasty and chordae tend to perform robotic endoscopic operations. Our operative outcomes suggest that index mitral valve surgery via a classic endoscopic approach yields similar clinical outcomes when compared to robotic endoscopic surgery. There was no difference in mortality across either cohort. Robotic endoscopic surgery was found to be associated with a significant increase in CPB duration. Freedom from moderate or severe mitral regurgitation or mitral valve replacement at last echocardiogram did not significantly differ between the two cohorts. A 2010 study of minimally invasive mitral surgery based on the STS database documented a twofold increase in the risk of stroke with less invasive mitral surgery but did not observe any difference in robotic endoscopic vs. non-robotic endoscopic minimally invasive surgery (12). This is thought to be due to retrograde systemic perfusion, and many centers including our own now use systematic CT imaging of the entire thoracic and visceral vasculature to screen out patients with significant aortic calcification and penetrating ulceration. We anticipate further reduction in our rate of clinical stroke, noting a stroke rate of zero percent annually after 2016 in either cohort.
Our study is potentially limited by several factors. This report describes the experience of a high-volume institution, and it may be difficult to extrapolate these results to other centers. This is a retrospective report and we have limited long-term follow-up. We have described short-term outcomes without comparing patients undergoing traditional median sternotomy. It is also possible that patients developed valvular degeneration outside of our follow-up period. Our analysis only included freedom from recurrent mitral regurgitation at the 10-year mark without further long-term data. Furthermore, patients with more challenging mitral pathology, including those with significant annular calcification, endocarditis, and other challenging pathologies are not well represented within our cohort as they may not be best served by minimally invasive techniques. Furthermore, while previous studies have demonstrated the safety and efficacy of concomitant procedures during minimally invasive mitral surgery, only one patient in our cohort underwent a concomitant tricuspid repair (16). Patients requiring atrial fibrillation ablation procedures, or tricuspid repairs at the time of mitral surgery may also undergo concomitant procedures via classic endoscopic or robotic endoscopic approaches.
Future studies should aim to focus on the long-term clinical and echocardiographic outcomes in patients who undergo robotic endoscopic repair, and repair rates should be routinely reported from institutional and multi-institutional studies. Our data reaffirms that both classic endoscopic and robotic endoscopic approaches allow repair of degenerative mitral valves with satisfactory short-term outcomes in a tertiary referral center.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108:II48-II54. [Crossref] [PubMed]
- Chitwood WR, Jr. Atlas of Robotic Cardiac Surgery. 1st edition. London: Springer-Verlag, 2014.
- Richardson L, Richardson M, Hunter S. Is a port-access mitral valve repair superior to the sternotomy approach in accelerating postoperative recovery? Interact Cardiovasc Thorac Surg 2008;7:678-83. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Bonatti J, Kiaii B, Alhan C, et al. The role of robotic technology in minimally invasive surgery for mitral valve disease. Expert Rev Med Devices 2021;18:955-70. [Crossref] [PubMed]
- Chitwood WR Jr, Rodriguez E, Chu MW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg 2008;136:436-41. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced "robotic" cardiac surgery: experience in 148 patients. J Thorac Cardiovasc Surg 2001;121:842-53. [Crossref] [PubMed]
- Folliguet T, Vanhuyse F, Constantino X, et al. Mitral valve repair robotic versus sternotomy. Eur J Cardiothorac Surg 2006;29:362-6. [Crossref] [PubMed]
- Murphy DA, Miller JS, Langford DA, et al. Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg 2006;132:776-81. [Crossref] [PubMed]
- Gammie JS, Chikwe J, Badhwar V, et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg 2018;106:716-27. [Crossref] [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10.
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [Crossref] [PubMed]
- Ko K, de Kroon TL, Post MC, et al. Minimally invasive mitral valve surgery: a systematic safety analysis. Open Heart 2020;7:e001393. [Crossref] [PubMed]
- Perier P, Hohenberger W, Lakew F, et al. Rate of repair in minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:751-7. [PubMed]
- Helmers MR, Kim ST, Altshuler P, et al. Mitral Valve Surgery in Pulmonary Hypertension Patients: Is Minimally Invasive Surgery Safe? Ann Thorac Surg 2021;111:2012-9. [Crossref] [PubMed]






