Aortic root replacement in bicuspid versus tricuspid aortic valve patients
Introduction
Replacement of the aortic root is necessary in patients with aortic root aneurysms or acute aortic dissection type A (AADA) involving the aortic root, and can be either performed in combination with aortic valve replacement [as described by Bentall and De Bono (1)] or by a valve-sparing approach [using aortic valve reimplantation or remodeling technique (2,3)]. Approximately 1 out of 9 AADA patients is a carrier of a bicuspid aortic valve (BAV), the most common cardiac anomaly affecting up to 2% of the general population (4). In an elective setting the current guidelines treat BAV as an important risk factor for AADA and thoracic aortic aneurysm development, by setting the cut-off diameter for elective replacement 5 mm lower than in patients with a tricuspid aortic valve (TAV) (5). However, several studies have raised doubts about the utilization of the absolute aortic diameter as an ideal risk marker for patients with either BAV or TAV (6).
Elective aortic root replacement is associated with excellent short- and long-term results (7,8) and, as recently published, does not increase the perioperative risk in emergency AADA surgery (9). Little data exists on comparison between BAV and TAV patients undergoing modified Bentall surgery. Our aim was to therefore compare patients with a BAV and TAV receiving either elective (group A) or emergency (group B) concomitant replacement of the aortic valve and root as a modified Bentall procedure. Our data may serve as contemporary benchmark for high-volume expert centers.
Methods
Patient selection
The study was approved by the ethics committee of the medical faculty of the University of Leipzig (#177/15). We retrospectively reviewed our institutional database and included all patients ≥18 years who underwent (I) elective modified Bentall surgery or (II) a modified Bentall procedure in case of AADA at our institution between 2000 and 2018. Exclusion criteria were previous cardiac or aortic surgery, known hereditary connective tissue disorder (e.g., Marfan syndrome, Ehlers-Danlos syndrome), surgery for acute endocarditis, prior cardiac or aortic surgery, and patients without sufficient data about the aortic valve morphology.
All patient charts, echocardiographic data and computed tomography (CT) scans were reviewed by two examiners. Aortic diameter was determined by contrast enhanced CT for aortic root and ascending aorta at the level of the pulmonary artery bifurcation.
Operative technique
All operations were performed via either full median sternotomy or upper J- or T-shaped hemi-sternotomy at the level of the 3rd or 4th intercostal space. In elective cases, cardiopulmonary bypass (CPB) was usually established via distal ascending aorta and right atrium cannulation. In the AADA group, arterial cannulation was performed via axillary cannulation or, infrequently, via femoral cannulation, and venous access was gained via direct cannulation of the atrial appendage. A left ventricular vent was used in all operations, usually via the right superior pulmonary vein. Antegrade application of crystalloid or blood cardioplegia was conducted in most cases, with retrograde cardioplegia used in select patients. In patients with moderate or severe aortic insufficiency, cardioplegia was usually administered directly into the coronary ostia using mushroom- or olive-tipped catheters. According to patient’s age and risk profile, standard biological prostheses sewn into a tubular dacron prostheses, xeno- or homograft root prostheses, or commercially available mechanically-valved conduits were used for root replacement. The modified Bentall procedure, routinely utilized at our institution, was just recently described by Khachatryan et al. (10).
Statistical analysis
Statistical analysis was performed using R version 4.1.2. (The R Foundation for Statistical Computing), and RStudio 4.1.2. (RStudio: Integrated Development Environment for R, PBC, Boston, USA). Continuous variables were expressed as median and interquartile range (IQR), categorical data presented as counts and percentages throughout the manuscript. Distribution of continuous variables was controlled by means of Shapiro-Wilk test and QQ-plots. Unmatched groups were compared using the Wilcoxon sum rank test, two-sided Fisher’s exact test, or Chi-square test, as appropriate.
In the elective cohort, propensity score matching was performed using 1:1 nearest neighbour method with 0.2 calliper. The following covariates were used for the matching in the elective cases: age, gender, body mass index, arterial hypertension, hyperlipidemia, diabetes mellitus, history of smoking, chronic obstructive pulmonary disease (COPD), peripheral arterial disease, coronary artery disease, prior myocardial infarction, prior stroke, preoperative glomerular filtration rate (GFR), preoperative left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class III–IV heart failure prior to the surgery, American Society of Anesthesiologists (ASA) class, type of the conduit used for the Bentall procedure (mechanical, biological or xeno-/homograft conduit), minimally invasive approach, extension of the aortic replacement [isolated ascending aortic replacement (AAR), hemiarch procedure or extended aortic arch surgery], aortic root enlargement, Morrow procedure, coronary artery bypass grafting (CABG) for coronary artery disease, mitral valve (MV) replacement or repair, other concomitant procedures, type of cardioplegia (blood, crystalloid, antegrade, retrograde or combined), displayed in Figure S1.
In the AADA cases, genetic matching (a form of nearest neighbour matching using generalized Mahalanobis distance) was performed in 1:1 fashion. The following covariates were used for the matching analysis in the AADA cases: age, gender, arterial hypertension, diabetes mellitus, history of smoking, COPD, peripheral arterial disease, coronary artery disease, prior stroke, chronic kidney disease, moderate or severe aortic stenosis (AS), NYHA III–IV class heart failure prior to surgery, preoperative LVEF, type of the conduit used for the Bentall procedure, preoperative malperfusion (coronary, cerebral, visceral or extremity malperfusion), CABG for coronary artery disease, and extended aortic arch surgery.
The covariates included in the propensity score model and genetic matching are presented in Figures 1,2.
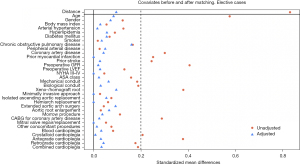
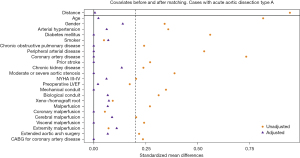
Missing values (not exceeding 8% of analyzed covariate) were replaced by means of multiple imputations based on Rubin’s rules. For comparison of the matched groups, we used the Wilcoxon-signed rank test for continuous, and McNemar’s test for categorical data. Statistical significance was set at a P value of ≤0.05 for two-tailed testing.
Results
Patient cohort
Elective Bentall
A total of 827 patients met the inclusion/exclusion criteria, of which 44% had a BAV and 56% a TAV anatomy. Bicuspid patients were approximately 7 years younger (P<0.001) and significantly healthier (less coronary artery disease, less prior stroke and chronic kidney disease) at the time of surgery. A total of 584 patients were successfully matched by 1:1 propensity score matching resulting in two matched groups with no differences in preoperative variables. Characteristics of the matched and unmatched cohort are displayed in Table 1. Covariates before and after propensity score matching are displayed in Figure 1.
Table 1
| Variables | Unmatched | Matched | SMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=827) | BAV (n=365, 44%) | TAV (n=462, 56%) | P value | Total (n=584) | BAV (n=292, 50%) | TAV (n=292, 50%) | P value | |||
| Age (years) | 62±16 | 58±18 | 65±14 | <0.001 | 62±15 | 61±16 | 62±18 | 0.28 | 0.084 | |
| Male gender | 658 [80] | 300 [82] | 358 [77] | 0.10 | 468 [80] | 238 [82] | 230 [79] | 0.38 | 0.072 | |
| BMI (kg/m2) | 27±5 | 27±5 | 27±5 | 0.43 | 27±5 | 27±5 | 27±5 | 0.62 | 0.028 | |
| Arterial hypertension | 646 [78] | 274 [75] | 372 [81] | 0.06 | 462 [79] | 230 [79] | 232 [79] | 0.84 | 0.016 | |
| Hyperlipidemia | 342 [41] | 142 [39] | 200 [43] | 0.20 | 246 [42] | 119 [41] | 127 [43] | 0.50 | 0.056 | |
| Diabetes mellitus | 98 [12] | 39 [11] | 59 [13] | 0.36 | 77 [13] | 36 [12] | 41 [14] | 0.52 | 0.055 | |
| History of smoking | 363 [44] | 162 [44] | 201 [43] | 0.80 | 245 [42] | 125 [43] | 120 [41] | 0.68 | 0.035 | |
| COPD | 38 [5] | 11[3] | 27 [6] | 0.05 | 30 [5] | 11 [4] | 19 [7] | 0.12 | 0.160 | |
| Peripheral arterial disease | 495 [60] | 221 [61] | 274 [59] | 0.72 | 345 [59] | 176 [60] | 169 [58] | 0.53 | 0.049 | |
| Coronary artery disease | 145 [18] | 44 [12] | 101 [22] | <0.001 | 76 [13] | 40 [14] | 36 [12] | 0.61 | 0.042 | |
| Prior myocardial infarction | 51 [6] | 13 [4] | 38 [8] | 0.005 | 24 [4] | 12 [4] | 12 [4] | 1.00 | 0.000 | |
| Prior stroke | 28 [3] | 6 [2] | 22 [5] | 0.01 | 11 [2] | 6 [2] | 5 [2] | 0.74 | 0.027 | |
| Preoperative GFR (mL/min/1.73 m2) | 95±42 | 102±44 | 90±42 | <0.001 | 98±42 | 97±43 | 98±40 | 0.87 | 0.020 | |
| Chronic kidney disease | 88 [11] | 27 [7] | 61 [13] | 0.007 | 54 [9] | 27 [9] | 27 [9] | 1.00 | – | |
| Prior dialysis | 1 [<1] | 0 [0] | 1 [<1] | 0.37 | 0 [0] | 0 [0] | 0 [0] | 1.00 | – | |
| Preoperative LVEF (%) | 60±14 | 60±11 | 60±15 | 0.13 | 60±13 | 60±12 | 60±13 | 0.60 | 0.027 | |
| NYHA class III–IV | 126 [5] | 59 [16] | 67 [15] | 0.51 | 90 [15] | 45 [15] | 45 [15] | 1.00 | 0.000 | |
| ASA class | 2±1 | 2±1 | 3±1 | 0.01 | 2±1 | 2±1 | 2±1 | 0.75 | 0.023 | |
Data expressed as n [%] or median ± IQR. BMI, body mass index; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association classification of heart failure; ASA, American Society of Anesthesiologists physical status classification; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference (presented for the matching covariates); IQR, interquartile range.
Emergency Bentall
A total of 258 patients admitted for AADA underwent a concomitant replacement of the aortic valve and aortic root, of which 15% had a BAV and 85% a TAV. Baseline characteristics displayed significant differences in age, with BAV patients being 13 years younger at time of dissection (49±17 vs. 62±19 years, P<0.001), previously known arterial hypertension (P=0.01), and preoperative aortic valve stenosis (P=0.005). Preoperative malperfusion rate was not different between bicuspid and tricuspid patients. After adjusting and matching 68 patients, 34 in each group, no differences remained (see Table 2). Covariates before and after propensity score matching are displayed in Figure 2.
Table 2
| Variables | Unmatched | Matched | SMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=258) | BAV (n=39, 15%) | TAV (n=219, 85%) | P value | Total (n=68) | BAV (n=34, 50%) | TAV (n=34, 50%) | P value | |||
| Age (years) | 60±20 | 49±17 | 62±19 | <0.001 | 50±17 | 49±18 | 51±15 | 0.90 | 0.014 | |
| Male | 167 [65] | 30 [77] | 137 [63] | 0.084 | 48 [71] | 25 [74] | 23 [68] | 0.53 | 0.129 | |
| DeBakey type I dissection | 179 [69] | 25 [64] | 154 [70] | 0.44 | 48 [71] | 24 [71] | 24 [71] | 1.00 | – | |
| Arterial hypertension | 209 [81] | 26 [67] | 183 [84] | 0.01 | 45 [66] | 23 [67] | 22 [65] | 0.74 | 0.062 | |
| History of smoking | 55 [21] | 9 [23] | 46 [21] | 0.83 | 13 [13] | 7 [21] | 6 [18] | 0.76 | 0.075 | |
| Coronary artery disease | 32 [12] | 1 [3] | 31 [14] | 0.060 | 2 [3] | 1 [3] | 1 [3] | 1.00 | 0.000 | |
| Prior myocardial infarction | 16 [6] | 1 [3] | 15 [7] | 0.48 | 1 [1] | 1 [3] | 0 [0] | 1.00 | – | |
| COPD | 15 [6] | 1 [3] | 14 [6] | 0.48 | 2 [3] | 1 [3] | 1 [3] | 1.00 | 0.000 | |
| Prior stroke | 16 [6] | 1 [3] | 15 [7] | 0.48 | 2 [3] | 1 [3] | 1 [3] | 1.00 | 0.000 | |
| Diabetes mellitus | 26 [10] | 1 [3] | 25 [11] | 0.14 | 2 [3] | 1 [3] | 1 [3] | 1.00 | 0.000 | |
| Peripheral arterial disease | 25 [10] | 1 [3] | 24 [11] | 0.14 | 1 [2] | 1 [3] | 0 [0] | 1.00 | 0.000 | |
| Chronic kidney disease | 89 [34] | 10 [26] | 79 [36] | 0.21 | 16 [24] | 9 [26] | 7 [21] | 0.41 | 0.139 | |
| Preoperative AR > moderate | 230 [89] | 35 [90] | 195 [89] | 1.00 | 61 [90] | 32 [94] | 29 [85] | 0.26 | – | |
| Preoperative AS > moderate | 29 [11] | 10 [26] | 19 [9] | 0.005 | 12 [18] | 6 [18] | 6 [18] | 1.00 | 0.000 | |
| NYHA III/IV | 114 [56] | 14 [36] | 100 [46] | 0.26 | 25 [37] | 13 [38] | 12 [35] | 0.78 | 0.061 | |
| Preoperative LVEF (%) | 55±10 | 60±9 | 55±10 | 0.09 | 55±7 | 59±7 | 55±8 | 0.52 | 0.024 | |
| Cardiopulmonary resuscitation | 20 [8] | 3 [8] | 17 [8] | 1.00 | 3 [4] | 3 [9] | 0 [0] | 0.25 | – | |
| Inotropic support | 55 [21] | 11 [28] | 44 [20] | 0.25 | 15 [22] | 10 [29] | 5 [15] | 0.10 | – | |
| Ventilation | 47 [18] | 9 [23] | 38 [17] | 0.38 | 15 [22] | 9 [27] | 6 [18] | 0.35 | – | |
| Pericardial effusion | 103 [40] | 16 [41] | 87 [40] | 0.88 | 26 [38] | 14 [42] | 12 [35] | 0.62 | – | |
| Malperfusion syndrome | 92 [36] | 10 [26] | 82 [37] | 0.16 | 17 [25] | 9 [26] | 8 [24] | 0.78 | 0.068 | |
| Cerebral malperfusion | 48 [19] | 5 [13] | 43 [20] | 0.38 | 9 [13] | 5 [15] | 4 [12] | 0.74 | 0.087 | |
| Coronary malperfusion | 37 [14] | 5 [13] | 32 [15] | 0.86 | 8 [12] | 4 [12] | 4 [12] | 1.00 | 0.000 | |
| Visceral malperfusion | 15 [6] | 1 [3] | 14 [6] | 0.48 | 2 [3] | 1 [3] | 1 [3] | 1.00 | 0.000 | |
| Extremity malperfusion | 24 [9] | 3 [8] | 21 [10] | 1.00 | 5 [7] | 3 [9] | 2 [6] | 0.56 | 0.113 | |
Data expressed as n [%] or median ± IQR. Cases with connective tissue disorders; and unknown type of aortic valve were not included. Categorical unmatched variables compared using Chi-square or Fisher test; continuous unmatched variables—by means of Wilcoxon sum rank test. In the matched cohort; McNemar’s test and Wilcoxon-signed rank test were used. AADA, acute aortic dissection type A; COPD, chronic obstructive pulmonary disease; AR, aortic regurgitation; AS, aortic stenosis; NYHA, New York Heart Association classification of heart failure; ASA, American Society of Anesthesiologists physical status classification; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference (presented for the matching covariates); IQR, interquartile range.
Operative details
Elective Bentall
Prior to matching, significantly more patients with a BAV received a mechanical valve conduit with a bigger prosthesis size (27±2 vs. 25±2, P=0.01) than TAV patients. Due to pre-existing co-morbidities, more concomitant CABG was performed in patients with TAV (P<0.001). Crystalloid cardioplegia was used significantly more often in BAV patients, although operative, CPB and aortic cross-clamp times were significantly longer in the TAV group before matching. After matching, no relevant differences remained between the two groups. All intraoperative details of the elective patients before and after matching are displayed in Table 3.
Table 3
| Variables | Unmatched | Matched | SMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=827) | BAV (n=365, 44%) | TAV (n=462, 56%) | P value | Total (n=584) | BAV (n=292, 50%) | TAV (n=292, 50%) | P value | |||
| Types of conduits | ||||||||||
| Mechanical valve conduit | 278 [34] | 141 [38] | 137 [30] | 0.007 | 191 [33] | 100 [34] | 91 [31] | 0.41 | 0.060 | |
| Biological valve conduit | 207 [25] | 109 [30] | 98 [21] | 0.004 | 168 [29] | 86 [29] | 82 [28] | 0.70 | 0.030 | |
| Xeno-/homograft root | 342 [41] | 115 [32] | 227 [49] | <0.001 | 225 [39] | 106 [36] | 119 [41] | 0.20 | 0.096 | |
| Prosthesis size (mm) | 27±2 | 27±2 | 25±2 | 0.01 | 27±2 | 27±2 | 27±2 | 0.02 | – | |
| Concomitant procedures | ||||||||||
| CABG for iatrogenic injury | 13 [2] | 2 [1] | 11 [2] | 0.04 | 5 [1] | 2 [1] | 3 [1] | 0.65 | 0.037 | |
| CABG for coronary artery disease | 113 [14] | 33 [9] | 80 [17] | 0.001 | 56 [10] | 29 [10] | 27 [9] | 0.78 | 0.024 | |
| Morrow procedure | 39 [5] | 13 [4] | 26 [6] | 0.16 | 28 [5] | 13 [4] | 15 [5] | 0.71 | 0.037 | |
| Aortic root enlargement | 3 [<1] | 1 [<1] | 2 [<1] | 1.00 | 3 [1] | 1 [<1] | 2 [1] | 0.56 | 0.066 | |
| MV repair or replacement | 1 [<1] | 0 [0] | 1 [<1] | 0.44 | 0 [0] | 0 [0] | 0 [0] | 1.00 | 0.000 | |
| Hemiarch | 12 [1] | 4 [1] | 8 [2] | 0.56 | 8 [1] | 4 [1] | 4 [1] | 1.00 | 0.000 | |
| Operative data | ||||||||||
| Minimally invasive approach | 83 [10] | 35 [10] | 48 [10] | 0.70 | 58 [10] | 29 [10] | 29 [10] | 1.00 | 0.000 | |
| CPB time (min) | 115±46 | 111±46 | 118±46 | <0.001 | 114±44 | 112±49 | 114±40 | 0.12 | – | |
| Cross-clamp time (min) | 88±34 | 87±31 | 89±35 | 0.03 | 88±33 | 88±32 | 88±32 | 0.07 | – | |
| Operative time (min) | 195±73 | 195±70 | 200±75 | 0.02 | 192±67 | 195±77 | 190±65 | 0.32 | – | |
| Cardioplegia type | ||||||||||
| Blood cardioplegia | 211 [26] | 86 [24] | 125 [27] | 0.25 | 136 [23] | 66 [23] | 70 [24] | 0.68 | 0.032 | |
| Crystalloid cardioplegia | 541 [65] | 258 [71] | 283 [61] | 0.005 | 411 [70] | 206 [71] | 205 [70] | 0.92 | 0.008 | |
| Antegrade cardioplegia | 662 [80] | 318 [87] | 344 [74] | <0.001 | 501 [86] | 249 [85] | 252 [86] | 0.70 | 0.031 | |
| Retrograde cardioplegia | 36 [4] | 10 [3] | 26 [6] | 0.043 | 18 [3] | 8 [3] | 10 [3] | 0.59 | 0.042 | |
| Combined cardioplegia | 54 [7] | 16 [4] | 38 [8] | 0.026 | 28 [5] | 15 [5] | 13 [4] | 0.69 | 0.034 | |
| Nonselective root cardioplegia | 9 [1] | 4 [1] | 5 [1] | 1.00 | 6 [1] | 4 [1] | 2 [<1] | 0.41 | – | |
| Cardioplegia unknown | 66 [8] | 17 [5] | 49 [11] | 0.002 | 31 [5] | 16 [5] | 15 [5] | 0.84 | – | |
Data expressed as n [%] or median ± IQR. CABG, coronary artery bypass grafting; MV, mitral valve, CPB, cardiopulmonary bypass; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference (presented for the matching covariates).
Emergency Bentall
As in the elective surgery cohort, significantly more BAV patients received a mechanical valved conduit compared to tricuspid patients prior to matching (54% vs. 37%, P=0.04). In the TAV group, more frozen elephant trunk procedures were performed (10% vs. 0%, P=0.05), resulting in a significantly longer circulatory arrest (CA) time (P=0.04) (see Table 4).
Table 4
| Variables | Unmatched | Matched | SMD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=258) | BAV (n=39, 15%) | TAV (n=219, 85%) | P value | Total (n=68) | BAV (n=34, 50%) | TAV (n=34, 50%) | P value | ||||
| Indication for Bentall procedure | |||||||||||
| Dissected root and/or coronary arteries | 170 [66] | 25 [64] | 145 [66] | 0.80 | 45 [66] | 23 [68] | 22 [65] | 0.81 | – | ||
| Calcified aortic valve | 14 [5] | 1 [3] | 13 [6] | 0.70 | 4 [6] | 0 [0] | 4 [12] | 0.13 | – | ||
| Severely dilated aortic root | 49 [19] | 9 [23] | 40 [18] | 0.51 | 9 [13] | 7 [21] | 2 [6] | 0.10 | – | ||
| Failure of supracoronary AAR or aortic valve sparing procedure | 9 [3] | 2 [5] | 7 [3] | 0.63 | 5 [7] | 2 [6] | 3 [9] | 0.65 | – | ||
| Unknown | 16 [6] | 2 [5] | 14 [6] | 1.00 | 5 [7] | 2 [6] | 3 [9] | 0.56 | – | ||
| Types of conduits | |||||||||||
| Mechanical valve conduit | 101 [39] | 21 [54] | 80 [37] | 0.04 | 36 [53] | 18 [53] | 18 [53] | 1.00 | 0.000 | ||
| Biological valve conduit | 96 [37] | 10 [26] | 86 [39] | 0.10 | 17 [25] | 9 [26] | 8 [24] | 0.71 | 0.068 | ||
| Xeno-/homograft root | 61 [24] | 8 [21] | 53 [24] | 0.69 | 15 [22] | 7 [21] | 8 [24] | 0.56 | 0.071 | ||
| Prosthesis size (mm) | 25±2 | 25±2 | 25±2 | 0.06 | 25±2 | 25±2 | 24±2 | 0.47 | – | ||
| Concomitant procedures | |||||||||||
| CABG (total) | 54 [21] | 2 [5] | 52 [24] | 0.008 | 7 [10] | 1 [3] | 6 [18] | 0.13 | – | ||
| CABG for coronary artery disease | 10 [4] | 0 [0] | 10 [5] | 0.37 | 0 [0] | 0 [0] | 0 [0] | 1.00 | 0.000 | ||
| MV repair | 1 [<1] | 1 [3] | 0 [0] | 0.15 | 1 [1] | 1 [3] | 0 [0] | 1.00 | – | ||
| Extent of distal aortic resection | |||||||||||
| Isolated AAR | 21 [8] | 3 [8] | 18 [8] | 1.00 | 5 [7] | 2 [6] | 3 [9] | 0.65 | – | ||
| Hemiarch | 150 [58] | 26 [67] | 124 [57] | 0.24 | 42 [62] | 22 [65] | 20 [59] | 0.53 | – | ||
| Total arch | 24 [9] | 5 [13] | 19 [9] | 0.38 | 6 [9] | 5 [15] | 1 [3] | 0.10 | – | ||
| Total arch and DTA | 3 [1] | 1 [3] | 2 [1] | 0.39 | 1 [1] | 1 [3] | 0 [0] | 1.00 | – | ||
| Elephant trunk | 41 [16] | 5 [13] | 36 [16] | 0.81 | 12 [18] | 5 [15] | 7 [21] | 0.48 | – | ||
| Frozen elephant trunk | 22 [9] | 0 [0] | 22 [10] | 0.05 | 3 [4] | 0 [0] | 3 [9] | 0.25 | – | ||
| Extended aortic arch surgery | 87 [34] | 10 [26] | 77 [35] | 0.25 | 21 [31] | 10 [29] | 11 [32] | 0.65 | 0.064 | ||
| Operative data | |||||||||||
| CPB time (min) | 201±84 | 194±55 | 202±89 | 0.51 | 197±98 | 195±51 | 203±125 | 0.17 | – | ||
| Aortic cross-clamp time (min) | 120±47 | 120±33 | 121±55 | 0.31 | 120±41 | 120±32 | 125±57 | 0.17 | – | ||
| CA time (min) | 25±22 | 21±14 | 26±6 | 0.04 | 24±17 | 24±15 | 23±22 | 0.23 | – | ||
| Operative time (min) | 325±140 | 310±80 | 325±154 | 0.53 | 315±102 | 308±84 | 327±157 | 0.21 | – | ||
| CA body temperature (℃) | 26±6 | 26±4 | 26±6 | 0.63 | 26±6 | 27±4 | 25±7 | 0.11 | – | ||
Data expressed as n [%] or median ± IQR. Categorical unmatched variables compared using Chi-square or Fisher test, continuous unmatched variables—by means of Wilcoxon sum rank test. In the matched cohort, McNemar’s test and Wilcoxon-signed rank test were used. AADA, acute aortic dissection type A; AAR, ascending aortic replacement; CABG, coronary artery bypass grafting; MV, mitral valve; DTA, descending thoracic aorta; CPB, cardiopulmonary bypass; CA, circulatory arrest; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference (presented for the matching covariates); IQR, interquartile range.
Postoperative outcomes
Elective Bentall
Prior to matching, more TAV patients had a postoperative cerebrovascular accidents and pulmonary complications, but both outcomes were not significantly different after matching. Thirty-day mortality was significantly higher in the tricuspid patients before matching (4% vs. 1%, P=0.05). Rate of in-hospital mortality was not different; this contrasts with the causes of death, which differed qualitatively (not formally tested due to low numbers). In the whole cohort, 4 out of 4 in-hospital deaths of BAV patients were due to low cardiac output, whereas of the 17 TAV patients that died in hospital, 57% were due to low cardiac output, 14% major cerebral injury, 14% sepsis, and 7% died of multiorgan failure. Intraoperative death, in-hospital mortality and 30-day mortality rates were not different between the groups. Length of hospital stay was similar after matching with 16 to 17 days in median (see Table 5).
Table 5
| Variables | Matched elective cohort | Matched type A dissection patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=584) | BAV (n=292, 50%) | TAV (n=292, 50%) | P value | Total (n=68) | BAV (n=34, 50%) | TAV (n=34, 50%) | P value | ||
| Complications | |||||||||
| Low cardiac output syndrome | 11 [2] | 8 [3] | 3 [1] | 0.13 | 7 [10] | 3 [9] | 4 [12] | 0.71 | |
| Perioperative myocardial infarction | 1 [<1] | 0 [0] | 1 [<1] | 1.00 | 2 [3] | 0 [0] | 2 [6] | 0.48 | |
| Stroke | 16 [3] | 7 [2] | 9 [3] | 0.62 | 2 [3] | 0 [0] | 2 [6] | 0.48 | |
| Re-exploration for bleeding | 44 [8] | 21 [7] | 23 [8] | 0.75 | 4 [6] | 0 [0] | 4 [12] | 0.13 | |
| Sepsis | 8 [1] | 4 [1] | 4 [1] | 1.00 | 18 [26] | 11 [32] | 7 [21] | 0.25 | |
| Gastrointestinal complications | 25 [4] | 11 [4] | 14 [5] | 0.55 | 2 [3] | 1 [3] | 1 [3] | 1.00 | |
| Respiratory failure | 45 [8] | 16 [5] | 29 [10] | 0.03* | 9 [13] | 4 [12] | 5 [15] | 0.74 | |
| Renal failure requiring dialysis | 16 [3] | 10 [3] | 6 [2] | 0.32 | 21 [31] | 7 [21] | 14 [41] | 0.09 | |
| Pacemaker implantation | 21 [4] | 14 [5] | 7 [2] | 0.13 | 10 [15] | 5 [15] | 5 [15] | 1.00 | |
| Hospital stay (days) | 10±6 | 10±6 | 11±6 | 0.75 | 16±11 | 16±11 | 16±10 | 0.86 | |
| Mortality | |||||||||
| Intraoperative death | 0 [0] | 0 [0] | 0 [0] | 1.00 | 3 [4] | 1 [3] | 2 [6] | 0.62 | |
| In-hospital mortality | 6 [1] | 4 [1] | 2 [1] | 0.41 | 13 [19] | 6 [18] | 7 [21] | 0.71 | |
| 30-day mortality | 10 [2] | 5 [2] | 5 [2] | 1.00 | 15 [22] | 8 [24] | 7 [21] | 0.71 | |
Data expressed as n [%] or median ± IQR. *, odds ratio 0.480 (95% confidence interval 0.220–0.991). BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; IQR, interquartile range.
Emergency Bentall
In emergency cases, postoperative respiratory failure was present in nearly half of the patients with TAV, compared to 21% in BAV patients (P=0.003). Although in-hospital mortality was not different with 15% and 17% respectively (P=1.0), the causes varied qualitatively between the groups (not formally tested due to low numbers). In the tricuspid group low cardiac output was the cause of death most frequently, whereas bicuspid patients more frequently died from multiorgan failure (1/6 vs. 4/38) or major cerebral injury (3/6 vs. 13/38). Due to the small patient numbers in the BAV group, these differences cannot be statistically analyzed. However, after matching, all postoperative outcome variables were not significantly different (see Table 5). Prior to matching, BAV patients had a 2-day shorter length of stay in the hospital, but after matching, both patient groups were similar with a median of 16 days.
Subgroup analysis of elective patients with aortic diameter <55 mm
Preoperative characteristics before and after matching were similar to the whole group—co-variates before and after matching are displayed in Figure S2. All significant intraoperative differences from the original cohort (see Table S1) were equalized after matching the patients. Outcome variables also showed no difference with very low in-house (1%) and 30-day mortality (2%). Before matching, rates of re-exploration for bleeding and respiratory failure were significantly higher in TAV subgroup; after matching, no differences could be detected. For all details see Table S2.
Discussion
In the current study, we compared perioperative outcomes in BAV and TAV patients undergoing elective and emergency modified Bentall surgery. After adjusting for preoperative characteristics, statistically significant differences in early outcomes were observed only with regards to respiratory failure rates in elective Bentall procedures. Prior to propensity matching, the group of BAV patients was younger and healthier at time of surgery. After matching, however, the two groups of patients were comparable with regards to all preoperative variables.
Over the past few decades, awareness for aortic root aneurysmal disease has increased resulting in timely preventive surgical intervention in root aneurysm patients and, in most AADA cases, immediate referral to emergency surgery (11). Outcomes of elective and emergency aortic root replacement have improved over time, resulting in decrease of mortality rates to about 3% in elective cases (12,13) and approximately 10–30% in emergencies, with higher mortality rates in AADA patients presenting with preoperative organ malperfusion (14,15). In our cohort of elective patients, 30-day mortality was 2% in both groups after matching, slightly lower than that reported in a large meta-analysis including 46 studies and 7,629 patients (16). This very low mortality rate in our high-volume center is reflective of the known association between center volume and outcomes in aortic surgery (17).
The analyzed group of AADA patients displayed a mortality rate of 28% in the current series, with no significant differences between the groups before and after matching. We have previously reported similar findings in these high-risk patients (18), as have several other large German centers with aortic expertise (19). As no difference in organ malperfusion [one of the major determinants of outcomes in AADA (15)] was present preoperatively between our two patient groups, no significant outcome differences could be detected. It has been shown in a study by Yang et al. that operative mortality is higher in AADA patients receiving modified Bentall surgery compared to David procedure, probably due to patient selection (20). Patients receiving modified Bentall tend to be in worse condition preoperatively, leading to higher in-hospital mortality.
Patients with a BAV are known to receive surgery approximately 10 years earlier compared to the tricuspid peers (21). In our retrospective analysis, BAV patients were 7 years younger in the group of elective modified Bentall surgery and 13 years younger in the emergency group (see Tables 1,2), leading to a different risk profile including less co-morbidities (e.g., chronic kidney disease, prior stroke, prior myocardial infarction). Respiratory failure after cardiac surgery is a well-known major adverse event, and its risk factors are critical preoperative state, poor left ventricular function, COPD, age and others (22). Especially in an acute setting, age itself is an important risk factor associated with higher mortality and morbidity (23,24). Hsu et al. analyzed nearly 4,000 AADA patients in Taiwan and found a respiratory failure rate of 29.1% in AADA patients at the age of 80 years and older vs. 17.2% in non-octogenarians (25). Similar results were observed in the present study. In the AADA group, respiratory failure was more prevalent in TAV patients (before matching: 46% vs. 21%, P=0.003 and after matching: 41% vs. 21%, P=0.09) despite similar operating times. This difference did not reach statistical significance in the matched cohort. Higher respiratory failure rates in TAV patients were, however, statistically significant in elective cases (12% vs. 5%, P<0.001 before matching, and 10% vs. 5%, P=0.03 after matching). This could be explained by the older and sicker TAV patients that even after matching, presented with a slightly higher rate of COPD, compared to BAV patients.
In elective cases the higher incidence of severe co-morbidities resulted in significantly worse 30-day mortality (4% vs. 1%) before matching; after matching no mortality differences were present. It is important to mention that even though matching analyses were performed to compare the results of Bentall procedure in BAV and TAV groups, in real-life scenario these two categories of patients do differ dramatically, particularly because of the significant age difference. Because the Bentall procedure is based on aortic valve replacement, and not reimplantation or reconstruction, superior outcomes in BAV patients are not surprising. At the same time, these age and comorbidity differences may be not as relevant in the decision-making process when a valve-sparing procedure (technically more complex in BAV) is to be performed. The increased complexity of repair and high rate of aortic valve stenosis in BAV patients are the main reasons that a consistently small number of David procedures are reported in this population.
BAV patients are being increasingly considered for transcatheter aortic valve replacement (TAVR) therapy, often with suboptimal results due to technical complexities (e.g., coronary anomalies, heavy calcification of the valve, elliptical annulus shape) associated with BAV (26). In contrast, the herein presented data demonstrates equally impressive results between BAV and TAV patients undergoing the modified Bentall procedure. A significant portion of the included patients (i.e., patients with aortic diameter <55 mm) may also fall into an area where TAVR could be considered as a possible therapeutic option. For these patients, as demonstrated in our subgroup analysis, Bentall results are excellent and not associated with higher complication rates when compared to TAV patients.
The pathological risk of the presence of a BAV has been more frequently investigated over the past decade, impacting guidelines for aortic replacement and shifting the absolute aortic diameter as indicator for aortic surgery between 45 and 50 mm for this specific patient group (5). Studies have demonstrated that every ninth AADA patient is a carrier of a BAV and that the dissection entry is more often located in the aortic root leading to a more extensive surgical repair (18). Apparently, no difference in incidence of rupture or dissection between BAV and TAV patients has been detected in the past (21). However, controversies exist on diameter at time of AADA in BAV patients. In 2013, Eleid et al. reported that the mean aortic diameter of BAV patients was 1 cm larger than their tricuspid peers (66±15 vs. 56±11 mm) (6), whereas a recent collaboration between Freiburg and the University of Pennsylvania showed that 76% of BAV patients had a diameter <5 cm (27). Furthermore, the presence of AS is associated with a higher risk for aortic rupture, dissection and death before operative repair with BAV patients (21). In our unmatched AADA patients, BAV carriers presented significantly more often with severe AS at the time of dissection. Furthermore, aortic disease in BAV patients is oftentimes limited to the proximal aorta, whereas in TAV patients the aorta is diseased as a whole—underlined by the higher rate of frozen elephant trunk procedures in the herein presented TAV patients (10% vs. 0%, P=0.05).
Our findings show that the early outcome of surgery is almost not different in patients with divergent aortic valve morphology, particularly when they are matched for preoperative characteristics. However, BAV patients present at a younger age with less comorbidities when receiving their aortic repair, and therefore tend to have better results in the unmatched cohort. For this reason, it is essential to establish a proper follow-up program monitoring for echocardiographic and CT controls over an extended time span in BAV patients. For TAV patients, CT imaging of the remaining aorta, especially after AADA, is key as in these patients, aortic disease is not limited to the proximal aortic segment, rather involving the aorta in its entirety. We have previously demonstrated that AADA patients undergoing retrograde perfusion (femoral-femoral cannulation) during AADA repair is associated with worse 10-year survival, compared to antegrade perfusion (71% vs. 51% survival at 10 years) (28) and general re-operation probability of AADA patients at 10 years has been previously published with 16% (29). These findings underscore the importance of long-term surveillance in TAV patients post-AADA repair.
Limitations
As this is a retrospective study data, could only be analyzed as documented. Patients with missing aortic valve morphology had to be excluded. The total number of patients with a documented BAV and AADA was relatively small. Finally, the current study focusses on early mortality and perioperative complications; follow-up data is not presented.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Ouzounian M, Feindel CM, Manlhiot C, et al. Valve-sparing root replacement in patients with bicuspid versus tricuspid aortic valves. J Thorac Cardiovasc Surg 2019;158:1-9. [Crossref] [PubMed]
- Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg 2017;153:S65-71. [Crossref] [PubMed]
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83:81-5. [Crossref] [PubMed]
- Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg 2018;156:e41-74. [Crossref] [PubMed]
- Eleid MF, Forde I, Edwards WD, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart 2013;99:1668-74. [Crossref] [PubMed]
- Yamabe T, Zhao Y, Kurlansky PA, et al. Assessment of long-term outcomes: aortic valve reimplantation versus aortic valve and root replacement with biological valved conduit in aortic root aneurysm with tricuspid valve. Eur J Cardiothorac Surg 2021;59:658-65. [Crossref] [PubMed]
- Wallen T, Habertheuer A, Bavaria JE, et al. Elective Aortic Root Replacement in North America: Analysis of STS Adult Cardiac Surgery Database. Ann Thorac Surg 2019;107:1307-12. [Crossref] [PubMed]
- Beckmann E, Martens A, Kaufeld T, et al. Frozen elephant trunk in acute aortic type a dissection: risk analysis of concomitant root replacement. Eur J Cardiothorac Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Khachatryan Z, Leontyev S, Magomedov K, et al. Management of aortic root in type A dissection: Bentall approach. J Card Surg 2021;36:1779-85. [Crossref] [PubMed]
- Mullan CW, Mori M, Bin Mahmood SU, et al. Incidence and characteristics of hospitalization for proximal aortic surgery for acute syndromes and for aneurysms in the USA from 2005 to 2014. Eur J Cardiothorac Surg 2020;58:583-9. [Crossref] [PubMed]
- Etz CD, Homann TM, Silovitz D, et al. Long-term survival after the Bentall procedure in 206 patients with bicuspid aortic valve. Ann Thorac Surg 2007;84:1186-93; discussion 1193-4. [Crossref] [PubMed]
- Urbanski PP, Heinz N, Zhan X, et al. Modified bio-Bentall procedure: 10-year experience. Eur J Cardiothorac Surg 2010;37:1317-21. [Crossref] [PubMed]
- Pacini D, Leone A, Belotti LM, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2013;43:820-6. [Crossref] [PubMed]
- Leontyev S, Légaré JF, Borger MA, et al. Creation of a Scorecard to Predict In-Hospital Death in Patients Undergoing Operations for Acute Type A Aortic Dissection. Ann Thorac Surg 2016;101:1700-6. [Crossref] [PubMed]
- Mookhoek A, Korteland NM, Arabkhani B, et al. Bentall Procedure: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2016;101:1684-9. [Crossref] [PubMed]
- Mori M, Shioda K, Wang X, et al. Perioperative Risk Profiles and Volume-Outcome Relationships in Proximal Thoracic Aortic Surgery. Ann Thorac Surg 2018;106:1095-104. [Crossref] [PubMed]
- Etz CD, von Aspern K, Hoyer A, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg 2015;48:142-50. [Crossref] [PubMed]
- Beckmann E, Martens A, Alhadi FA, et al. Is Bentall Procedure Still the Gold Standard for Acute Aortic Dissection with Aortic Root Involvement? Thorac Cardiovasc Surg 2016;64:116-23. [PubMed]
- Yang B, Patel HJ, Sorek C, et al. Sixteen-Year Experience of David and Bentall Procedures in Acute Type A Aortic Dissection. Ann Thorac Surg 2018;105:779-84. [Crossref] [PubMed]
- Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338-44. [Crossref] [PubMed]
- Bailey ML, Richter SM, Mullany DV, et al. Risk factors and survival in patients with respiratory failure after cardiac operations. Ann Thorac Surg 2011;92:1573-9. [Crossref] [PubMed]
- Trimarchi S, Eagle KA, Nienaber CA, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg 2010;140:784-9. [Crossref] [PubMed]
- Rylski B, Suedkamp M, Beyersdorf F, et al. Outcome after surgery for acute aortic dissection type A in patients over 70 years: data analysis from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2011;40:435-40. [Crossref] [PubMed]
- Hsu ME, Chou AH, Cheng YT, et al. Outcomes of Acute Aortic Dissection Surgery in Octogenarians. J Am Heart Assoc 2020;9:e017147. [Crossref] [PubMed]
- Makkar RR, Yoon SH, Leon MB, et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA 2019;321:2193-202. [Crossref] [PubMed]
- Kreibich M, Rylski B, Czerny M, et al. Type A Aortic Dissection in Patients With Bicuspid Aortic Valve Aortopathy. Ann Thorac Surg 2020;109:94-100. [Crossref] [PubMed]
- Etz CD, von Aspern K, da Rocha E, Silva J, et al. Impact of perfusion strategy on outcome after repair for acute type a aortic dissection. Ann Thorac Surg 2014;97:78-85. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]






